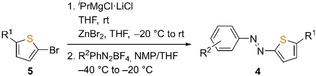
Table 1.
Synthesis of thiophenylazobenzenes 4 b–l using dithiophenylzinc reagents and aryldiazonium tetrafluoroborates (6) as building blocks.
|
| ||||
|---|---|---|---|---|
|
Entry |
R1 |
R2 |
Product |
Isolated yield [%] |
|
1 |
H |
H |
4 b |
59 |
|
2 |
H |
4‐OMe |
4 c |
42 |
|
3 |
H |
4‐CN |
4 d |
70 |
|
4 |
H |
4‐CF3 |
4 e |
61 |
|
5 |
H |
3‐OMe |
4 f |
20[a] |
|
6 |
H |
3‐CN |
4 g |
60 |
|
7 |
OMe |
H |
4 h |
24 |
|
8 |
OMe |
4‐CN |
4 i |
48 |
|
9 |
Me |
4‐CN |
4 j |
70 |
|
10 |
Me |
4‐CF3 |
4 k |
54 |
|
11 |
CN |
H |
4 l |
0.3[b] |
[a] The 3‐methoxybenzenediazonium salt 6 f was added neat in small portions to the thiophenylzinc reagent at −60 °C due to its instability in solution. [b] The Grignard reagent was directly added to the diazonium suspension at −80 °C.