Figure 1.
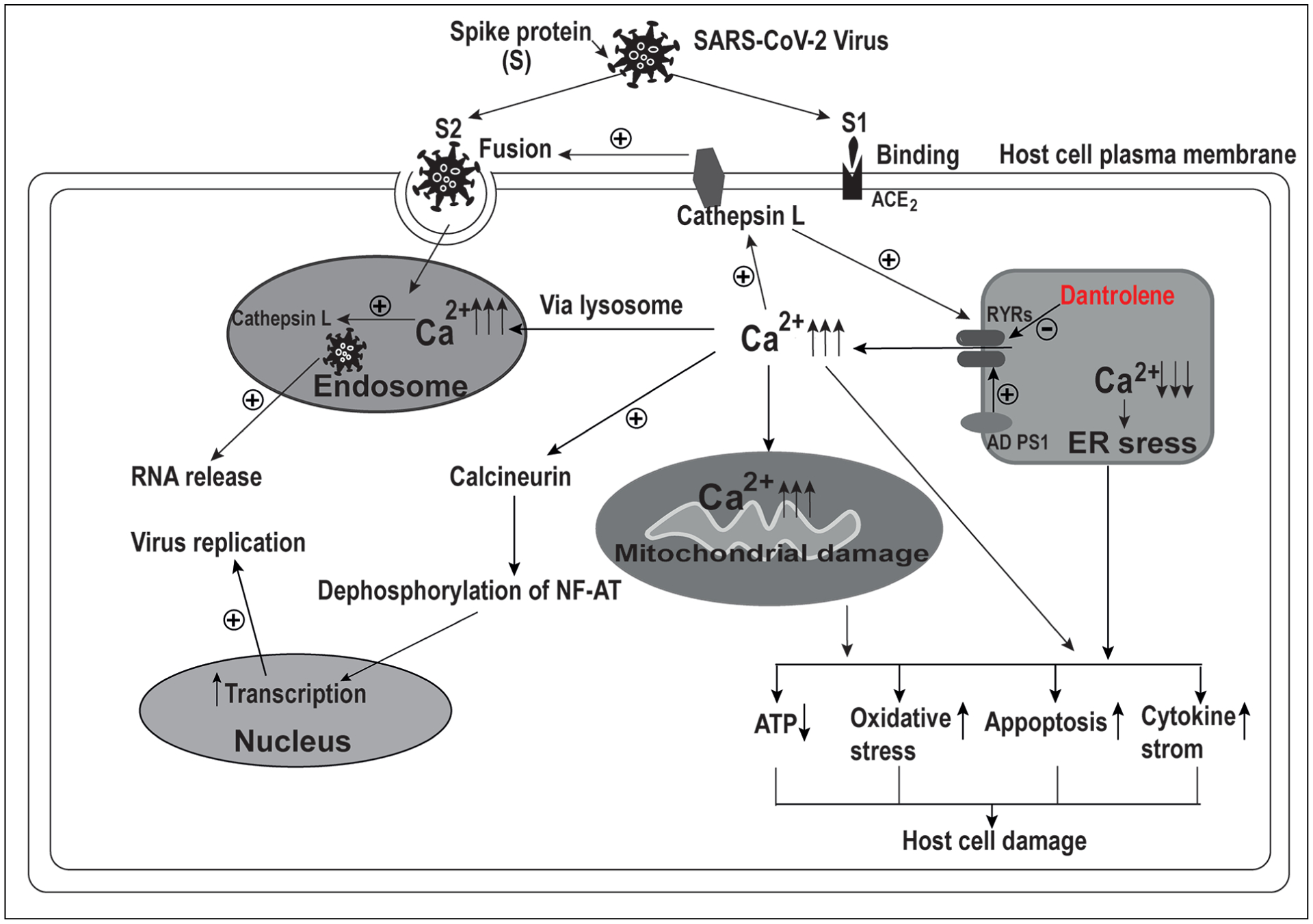
Dantrolene might inhibit infection and replication of SARS-CoV-2 and associated pathology. Cathepsin L, a protease on the plasma membrane of host cells, increases Ca2+ release from the endoplasmic reticulum (ER) via the ryanodine receptors (RyRs). The associated elevation of cytosolic Ca2+ concentration, in turn, increases cathepsin L activity. Cathepsin L promotes virus fusion with host cells by cleaving and activating the spike (S) protein. High levels of extracellular and cytosolic Ca2+ concentrations are also necessary for virus fusion and endocytosis. Cathepsin L in the endosome, under the condition of a high level of Ca2+ concentration, promotes virus RNA release into the cytosol. On the other hand, the increased cytosolic Ca2+ concentration due to the overactivation of RyRs activates calcineurin, which dephosphorylates NF-AT and translocates into the nucleus for promoting transcription and virus replication. Excess Ca2+ release from ER via overactivation of RyRs in AD cells results in depletion of ER Ca2+ and associated ER stress, as well as the overloading of mitochondria with Ca2+ and associated mitochondria damage. All of the above pathologies eventually result in impaired ATP production, oxidative stress damage, apoptosis, and cytokine storm, leading to final host cell damage. Dantrolene inhibits the infection and replication of the SARS-CoV-2 virus and host cell damage by inhibiting abnormal and excessive activation of RyRs and restoring the intracellular Ca2+ homeostasis.
