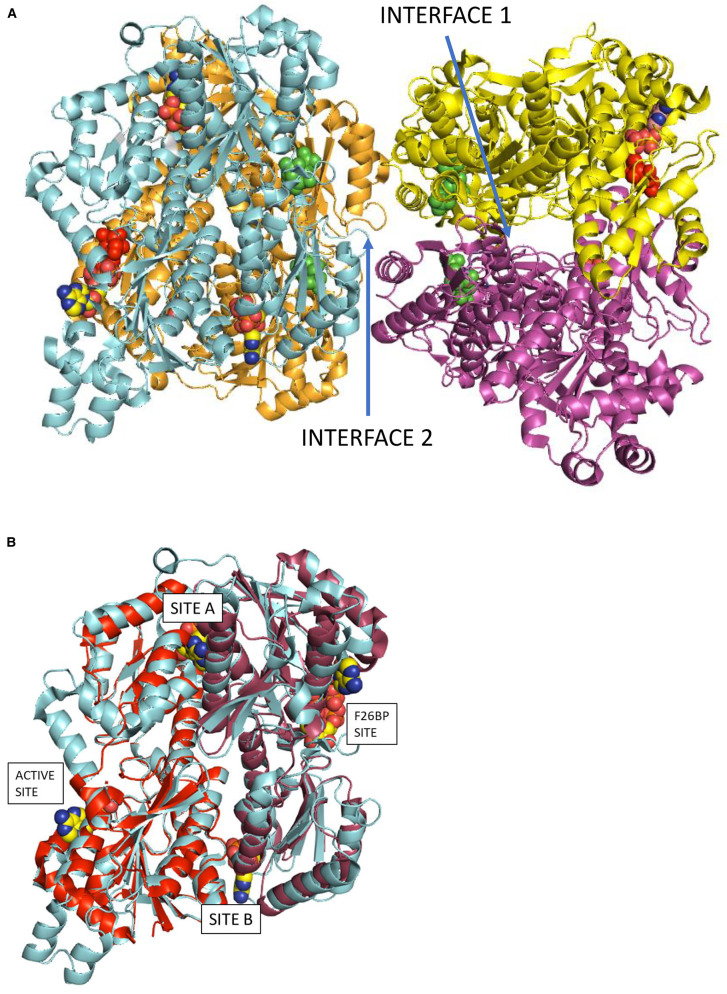
Figure 1. Models of PFK structures showing chain interface sites and allosteric binding sites.
Panel A shows the PFK-P tetrameric structure (PDB reference: 4XZ2) with the locations of the active sites and allosteric sites indicated. Interface residues between protomer pairs were identified using PDBePISA. Interface 1 is between chains A (orange) and B (cyan) (or C (purple) and D (yellow)). The view is along a pseudo 2-fold symmetry axis of the B/A dimer. There is an equivalent symmetry related ‘interface 1’ between chains C and D. Interface 2 is between chains B and C (or the symmetry related chains A and D). The active site of chain B shows F6P (red) and ADP (atom-type colour). The 4 molecules of F26BP in green are close to Interface 2. Two ADP molecules (atom-type colour) are modelled into the remaining two allosteric sites of chain B (cyan) labelled in Panel B as site A and site B. Panel B shows an overlay of EcPFK (PDB reference: 1PFK) and PFK-P (cyan). Chain A of EcPFK (1PFK) fitted onto residues 1–384 of chain B of PFK-P is red and chain B of EcPFK (1PFK) fitted onto residues 385–784 of chain B of PFK-P is raspberry. The allosteric binding sites A and B are occupied by ADP from the EcPFK X-ray structure.