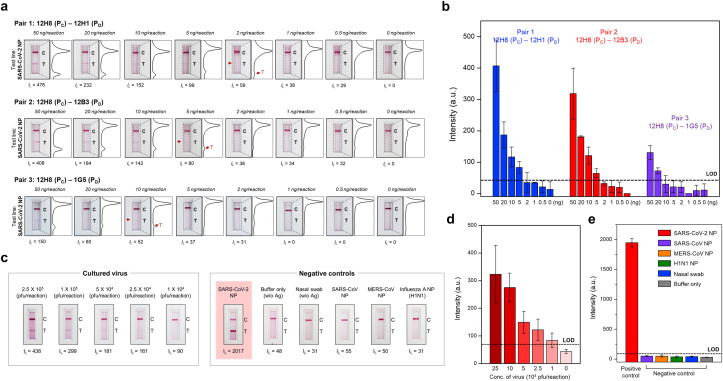
Fig. 5.
Sensitive and specific detection of the SARS-CoV-2 NP antigen. (a) Sensitivity of the sandwich LFIA for SARS-CoV-2 NP. Serially diluted SARS-CoV-2 NP (concentration range: 50 ng/reaction to 0.5 ng/reaction) was tested. After 20 min, the line intensities were analyzed using the portable LFIA reader (IL: line intensity) and the LFIA strips were photographed using a smartphone. In addition, the intensity of the test and control lines was converted to a peak histogram by an image analyzer. (b) Intensity of the test lines measured by the portable LFIA reader. The limit of detection was calculated as the mean value of the negative controls plus three times the standard deviation. The 12H8(PC)/12H1(PD) pair was the most sensitive for SARS-CoV-2 NP (as low as 2 ng of target antigen). (c) Sensitivity and selectivity of the best pair [12H8(PC)/12H1(PD)] for SARS-CoV-2 NP. Serially diluted cultured SARS-CoV-2 virus samples (concentration ranges: 2.5 × 105 pfu to 1 × 104 pfu) were used to evaluate the diagnostic performance of the LFIA biosensor. Moreover, NP from SARS-CoV, MERS-CoV, and influenza virus, or nasal swab specimens, were tested to investigate cross-reactivity (antigen concentration of all controls: 100 ng/reaction). After 20 min of sample flow, the intensities (IL) of test lines were measured using the portable LFIA reader and each LFIA strip was photographed using a smartphone. (d) Ability of the LFIA biosensor to detect cultured SARS-CoV-2. Using the best pair [12H8(PC)/12H1(PD)], the LFIA biosensor successfully detected cultured SARS-CoV-2 levels as low as 1 × 104 pfu. (e) Cross-reactivity of the proposed LFIA biosensor with various negative controls. There was no cross-reactivity with SARS-CoV, MERS, influenza NP, or nasal swab specimens (antigen concentration of all controls: 100 ng/reaction).