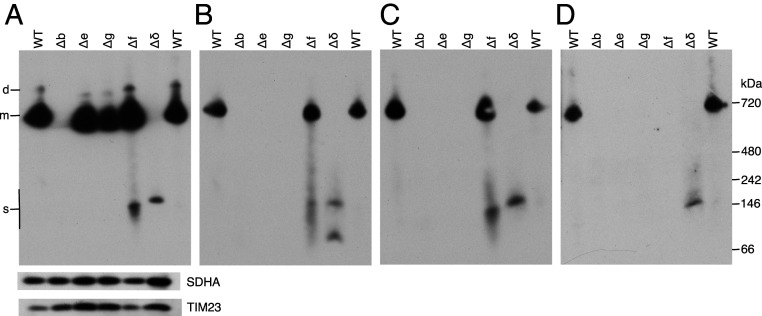
Fig. 5.
Oligomeric states of ATP synthase and vestigial forms in HAP1-WT, HAP1-∆b, HAP1-∆e, HAP1-∆g, HAP1-∆f, and HAP1-∆δ cells. ATP synthase and vestigial complexes were extracted from mitoplasts with digitonin (12 g/g protein). Extracts were fractioned by CN-PAGE, and complexes detected with antibodies against individual subunits of ATP synthase. (A), subunit b. (B), subunit e. The lower band in the ∆δ lane is probably free subunit e. (C), subunit g. (D), subunit f. The SDHA subunit of complex II and TIM23 are loading controls. On the left, d, and m, dimeric, and monomeric ATP synthase, respectively; s, subcomplex b-e-g-f in Δδ samples and b-e-g in HAP1-∆f samples. The positions of protein markers are shown on the right.