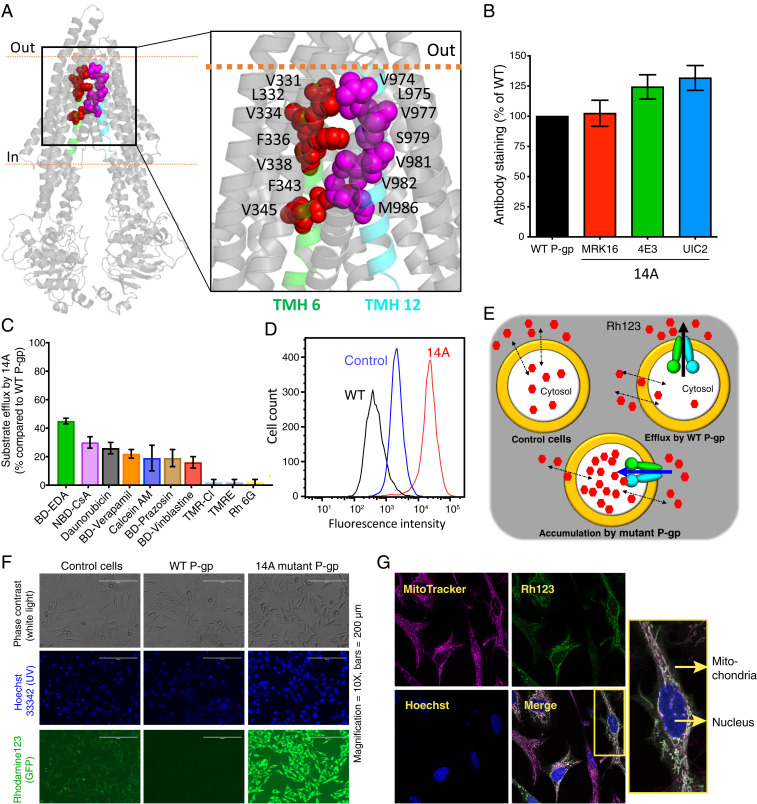
Fig. 1.
Transport direction is reversed by mutations in TMHs 6 and 12 of P-gp. (A) Inward open human P-gp structure (PDB ID: 6QEX) highlighting the location of residues mutated in 14A. The residues selected for mutation in TMH 6 and TMH 12 are shown as red and magenta spheres, respectively. (B) Relative surface expression of 14A detected by human P-gp–specific antibodies MRK-16, 4E3, and UIC2, with binding to WT P-gp taken as 100%. (C) Relative efflux of various substrates by 14A, with efflux by WT P-gp taken as 100%. Although 25 substrates (list given in ref. 10) were tested, results for only the top 10 are shown (mean ± SD, n ≥ 3). (D) A typical histogram showing uptake of Rh123 by 14A. Untransduced cells were used as a control for equilibration (referred to as control cells here and in subsequent figures), and Rh123 efflux from WT P-gp–expressing cells is shown for comparison. (E) Cartoon representation of substrate uptake by 14A as compared to efflux by WT protein. (F) Accumulation of Rh123 was determined by fluorescence microscopy. In the top row are phase contrast images, in the middle row are nuclei stained with Hoechst dye, and the bottom row of images show Rh123 fluorescence signal. (G) Confocal microscopy of HeLa cells expressing 14A after uptake of Rh123 (green). MitoTracker (pink), and Hoechst (blue) are markers for mitochondria and nuclei, respectively. The merged image shows colocalized sections (white), and a single cell is enlarged in the inset. GFP, green fluorescent protein; TMR-CI, tetramethylrosamine chloride; TMRE, tetramethylrhodamine, ethyl ester.