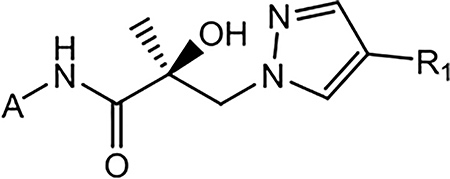
Table 3.
In Vitro AR Activity of 21a–21j (Series II)
| ID (R1) | Structure of the A-ring (A) |
Binding (Ki) / Transactivation (IC50) (μM) | SARD Activity (% degradation) | F.L. DC50 (μM) | ||
|---|---|---|---|---|---|---|
| Ki (DHT = 1 nM)a | IC50a | Full Lengtha (LNCaP) at 1 μM | Splice Varianta (22RV1) at 10 μM | |||
| 21a (4-F) | 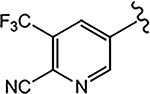 |
>10 | 0.062 | 54 | 81 | 0.88 |
| 21b (4-CF3) | 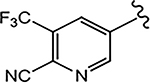 |
2.286 | 0.208 | 10 | N.A.b | - |
| 21c (4-CN) | 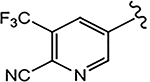 |
0.089 | 0.059 | 10 | N.A.b | - |
| 21d (4-NHCOOtBu) | 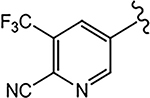 |
>10 | 6.108 | - | N.A.b | - |
| 21e (4-F) | 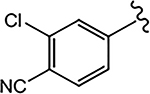 |
>10 | 0.427 | 42 | 0 | - |
| 21f (4-F) | 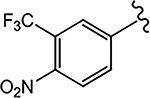 |
N.A.b | Partial Agonist | N.A.b | N.A.b | - |
| 21g (4-F) | 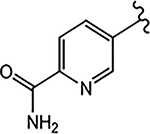 |
>10 | No effect | 0 | N.A.b | |
| 21h (4-F) | 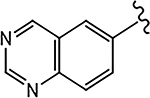 |
>10 | No effect | N.A. | N.A. | - |
| 21i (4-F) | 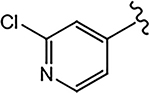 |
N.A. | 2.470 | 75 | N.A. | N.A. |
| 21j (4-F) | 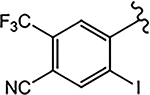 |
N.A. | 5.450 | N.A.b | N.A. | - |
AR binding, transactivation, and degradation assays were performed and values are reported as described in Table 2.
N.A. indicates data not available.