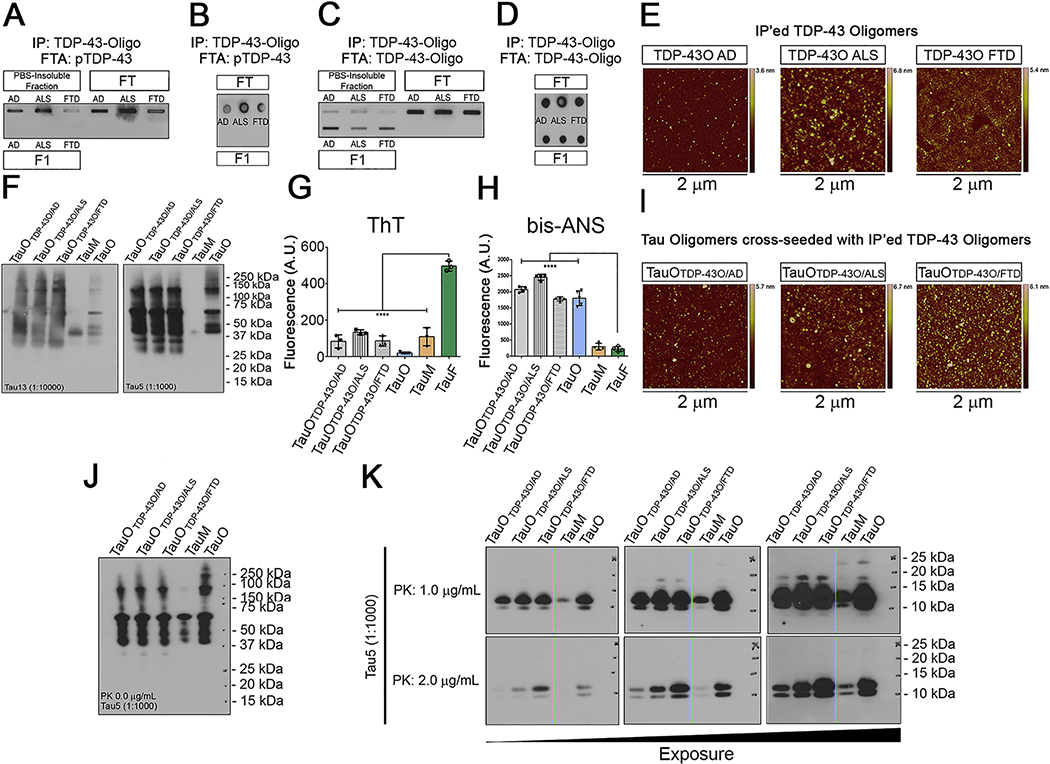
Figure 4. TDP-43O seeding properties.
(A) Filter Trap assay of PBS-insoluble, FT, and IP’ed BDT43Os (F1) and immunoblotted (IB) with the pTDP-43 antibody. (B) Dot blot of FT and F1 BDT43Os and IB with the pTDP-43 antibody. (C) Filter Trap of PBS-insoluble, FT, and IP’ed BDT43Os (F1) and IB with the TDP-43O antibody (D) Dot blot of IP (FT and F1) BDT43O and IB with TDP-43O antibody. (E) Representative AFM images of IP’ed BDT43Os from AD, ALS and FTD. (F) Tau13 and Tau5 IB of BDT43O (AD, ALS, and FTD) cross-seeded with TauM. TauM and TauO were used as controls (Lanes 4 and 5, respectively). (G) Fluorescence intensity measurement of ThT for BDT43Os and controls (TauO, TauM, and TauF). ThT spectroscopic analyses showed that hydrophobic BDT43Os and control TauO and TauM have significantly less binding affinity for ThT than TauF (one-way ANOVA, Dunnett’s multiple comparisons test ****p<0.001). (H) Fluorescence intensity measurement of bis-ANS to BDT43Os and controls (TauO, TauM, and TauF). Bis-ANS spectroscopic analyses showed that hydrophobic BDT43Os and TauO have significantly higher binding affinity for bis-ANS than TauF and TauM (one-way ANOVA, Dunnett’s multiple comparisons test ****p<0.001). (I) Representative AFM images of TauO cross-seeded with IP’ed BDT43Os from AD, ALS and FTD. (J) Tau5 IB of 0 μg/mL PK BDT43O (AD, ALS, and FTD) cross-seeded with TauM; TauM and TauO were used as controls (Lanes 4 and 5, respectively). (K) Tau5 IB of 1.0 (top) and 2.0 (bottom) μg/mL PK BDT43O (AD, ALS, and FTD) cross-seeded with TauM; TauM and TauO were used as controls (Lanes 4 and 5, respectively). The three immunoblots presented are different exposures of the same immunoblot.