Abstract
Influenza, a common cause of acute respiratory infections, is an important health problem worldwide, including in India. Influenza is associated with several complications; people with comorbidities and the elderly are at a higher risk for such complications. Moreover, the influenza virus constantly changes genetically, thereby worsening therapeutic outcomes. Vaccination is an effective measure for the prevention of influenza. Despite the availability of global guidelines on influenza vaccination in adults, country-specific guidelines based on regional variation in disease burden are required for better disease management in India. With this aim, the Indian Chest Society and National College of Chest Physicians of India jointly conducted an expert meeting in January 2019. The discussion was aimed at delineating evidence-based recommendations on adult influenza vaccination in India. The present article discusses expert recommendations on clinical practice guidelines to be followed in India for adult influenza vaccination, for better management of the disease burden.
KEY WORDS: Adult vaccination, India, consensus-based recommendations, guidelines, influenza, vaccination timing, live attenuated influenza vaccine, inactivated influenza vaccine
EXECUTIVE SUMMARY
Influenza is a communicable but preventable respiratory viral disease that mainly affects the nose, throat, and bronchi. Global estimates indicate that severe influenza is associated with over 600,000 deaths annually. India has not been spared by the illness; however, there is a dearth of robust data on the influenza burden in the country. Estimates from individual studies in the past decade reveal a case fatality rate of 7.5%. The influenza virus changes genetically through antigenic shift and drift, which in turn affects the severity of the disease. Vaccination is the most effective method to prevent and contain influenza outbreaks. Various types of influenza vaccine (live, attenuated, and recombinant) are available for preventing the disease. These vaccines are also safe and effective when administered concomitantly and sequentially with other inactivated or live vaccines. Vaccination is an indispensable preventive strategy against influenza, specifically for individuals who live with or care for high-risk individuals; older adults (>50 years); pregnant women; children; and individuals with chronic medical conditions. Although various global guidelines/recommendations are in place for influenza vaccination, specific Indian recommendations for clinicians practicing in the country are lacking.
INFLUENZA VACCINATION
-
What are the various vaccines available for influenza in India and how is the nasal influenza vaccine useful in the Indian clinical scenario?
-
For the prevention of influenza in India, two influenza vaccines are commercially available and recommended for clinical use:
- Standard-dose trivalent inactivated influenza vaccine (IIV)
- Standard-dose quadrivalent IIV.
-
Despite the availability of robust Indian safety data for the live attenuated influenza vaccine (LAIV) (nasal vaccine), the absence of robust evidence on the effectiveness of LAIV compared to IIV across various age groups in India, led the panel to recommend the use of IIV over LAIV.
- The panel added that LAIV can be used in special circumstances, based solely on the prescribing clinician's discretion. (Grade 3A)
-
-
Is there any preference for any specific vaccine over another among the various available influenza vaccines?
- Experts recommended the use of quadrivalent IIV over trivalent IIV in developing nations such as India (Grade 2A).
-
What are the contraindications and precautions for various influenza vaccines?
- Although influenza vaccines have a good safety profile, certain precautions and contraindications govern their use. IIV and LAIV should not be administered to patients with a history of severe allergic reaction to any components of the vaccine. Other contraindications are relative contraindications and will be discussed in the latter part of this document.
-
What are the recommendations concerning influenza vaccination for individuals with egg allergy and previous history of Guillain–Barré syndrome (GBS)?
-
Individuals with a history of egg allergy and who have:
- Experienced only urticaria (hives) after exposure to egg, can receive an age-appropriate influenza vaccine (IIV or LAIV) (Grade usual practice point [UPP])
- Experienced reactions to egg involving symptoms other than urticaria (hives), such as angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or another emergency medical intervention, can receive an age-appropriate influenza vaccine (IIV or LAIV) (Grade UPP)
- Experienced a severe reaction in the past, regardless of the suspected antigen, should not receive the vaccine.
- Consistent with the existing evidence, the experts recommended no requirement of a postvaccination observation period for individuals who are egg allergic (Grade UPP)
- The experts recommended against the use of influenza vaccination in individuals who are not at higher risk for severe influenza complications and are known to have experienced GBS within 6 weeks of previous influenza vaccination (Grade UPP).
-
-
What is the recommendation for influenza vaccination for patients on antiviral therapy?
- The experts recommended vaccination with IIV for individuals receiving antiviral medications either for treatment or chemoprophylaxis (Grade UPP).
-
What is the recommendation for influenza vaccination for patients on concomitant statin therapy?
- The experts acknowledged that statins have the propensity to reduce the effectiveness of the influenza vaccine, but nevertheless suggested the use of influenza vaccination in such patients (Grade 3A).
-
What is the recommendation for influenza vaccination for multiple sclerosis (MS) patients?
- The experts recommended a standard dose (SD) of IIV and avoidance of LAIV for MS patients (Grade UPP).
-
What is the recommendation for influenza vaccination for patients on corticosteroid therapy?
- The panel acknowledged that corticosteroid therapy reduces vaccine effectiveness, but suggested that long-term corticosteroid therapy in an individual should not preclude vaccine use in patients suffering from chronic pulmonary disease (Grade 2A).
-
What is the coadministration schedule of influenza vaccines with respect to other recommended vaccines?
- In line with the evidence, the experts accepted and recommended that IIV can be concomitantly or sequentially administered with other inactivated vaccines or with live vaccines (Grade 1A)
- For LAIVs, the experts recommended maintaining at least four weeks' gap between the administration of LAIV and another live vaccine (Grade UPP)
- LAIV does not interfere with the immune response of measles, mumps, and rubella (MMR) or varicella vaccines; hence, the experts recommended that they can be administered at the same visit (Grade 1A). However, the experts suggested that injectable vaccines that are given concomitantly with the influenza vaccine should be administered at separate anatomical sites (Grade UPP).
-
What are the recommended storage and handling specifications for various influenza vaccines?
-
The expert-recommended storage of the influenza vaccine is as follows:
- Only in the standout refrigerator, within a temperature range of 2°C–8°C (average temperature of 5°C) (Grade UPP)
- The vaccine should never be frozen or stored in the door of the refrigerator (Grade UPP). Household refrigerators are not recommended for storing the vaccine, due to their lack of temperature uniformity and due to the risk of contamination and power outages (Grade UPP)
- For transportation of influenza vaccines, the experts recommended the use of cold chains with immediate transfer of vaccines to a refrigerator with a uniform temperature range upon arrival. For storage of influenza vaccines in a portable storage unit, the temperature should be well-maintained and recorded (Grade UPP).
-
-
What is the recommended dose and frequency of influenza vaccination in India?
- As mentioned previously, standard-dose quadrivalent vaccine is preferred over trivalent vaccine for annual administration in individuals with appropriate indications (Grade 2A)
- The experts recommended a single HD of IIV, when available, for Indian clinical practice (Grade 1A)
- In view of the dearth of evidence comparing single-dose annual influenza vaccination and biannual influenza vaccination, the experts recommended annual influenza vaccination (Grade UPP).
-
What is the ideal timing for influenza vaccination in India?
- The experts acknowledged variations in the peak influenza season across various parts of the country. They recommended influenza vaccination from September to October for cities with temperate seasonality and April–May for cities with the peak monsoon season in July–September [Table 1 and Figure 1] (Grade UPP).
-
Are individual regional protocols for influenza vaccination required in India?
- The panel acknowledged the need for regional influenza vaccination and unanimously agreed on the need for in-depth national surveillance data to observe influenza seasonality in various Indian states. These data will further help formulate peak-seasonality-based recommendations for different regions of India (Grade UPP).
-
What are the recommendations for influenza vaccination for adults in India?
- The experts acknowledged the cost-effectiveness of influenza vaccination in India, specifically for adults >50 years. The recommendations specific to the India population proposed by the panel are listed in Table 2.
-
What are the recommendations for adults at high risk for influenza in India?
- Based on the evidence, the experts recommended routine influenza vaccination for adult Indians with specific medical conditions and indications that predispose to a high risk of complications due to influenza. The recommendations for influenza vaccination for high-risk adults are presented in Table 3.
-
What are the recommendations for influenza vaccination for travelers, Hajj pilgrims, and individuals attending mass gatherings?
- Influenza vaccination is recommended for individuals (irrespective of their vaccination status) traveling to parts of the world where influenza activity is ongoing. The administered influenza vaccination should consist of strains that are currently circulating at the travel destination. The vaccine is recommended to be administered 2 weeks before the scheduled departure (Grade UPP)
- The experts recommended influenza vaccination for Hajj pilgrims (Grade 2A) and individuals attending mass gatherings, irrespective of the influenza season at home or the place of visit. This particular recommendation is based on the fact that people coming from different geographical regions have different influenza seasonal activity. Therefore, such people can contract influenza infection irrespective of influenza seasonal activity at home or the place of visit (Grade UPP).
-
Is it justifiable to classify individuals into various priority risk groups for influenza vaccination in India?
- The experts accepted and agreed upon the categorization of high-risk groups for influenza laid down by the Ministry of Health and Family Welfare, India. They further suggested including veterinary, poultry workers, and military personnel in the high-risk group (Grade UPP).
Table 1.
Proposed vaccination timing for different cities in India
| Proposed vaccination timing | Cites |
|---|---|
| April-May | Delhi, Lucknow, Kolkata, Dibrugarh, Pune |
| September-October | Srinagar, Chennai, Vellore |
Figure 1.
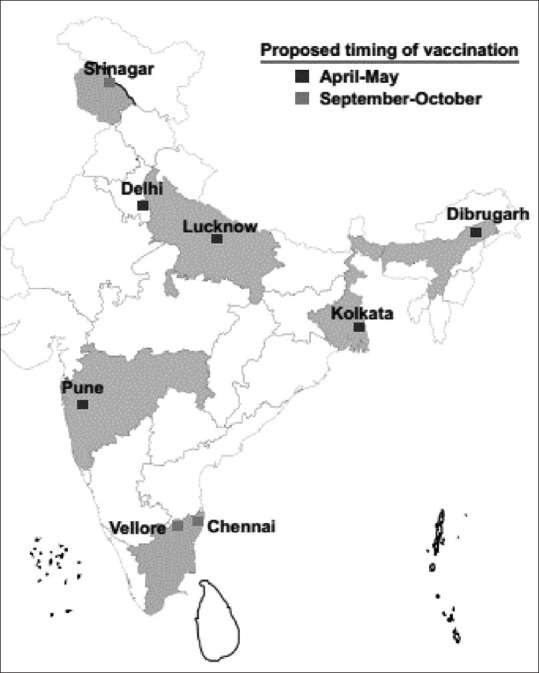
Proposed region-wise vaccination timing across different cities in India
Table 2.
Recommendations for influenza vaccination in the Indian population
| Age group (years) | Influenza vaccination recommendations |
|---|---|
| 18-49 | 1 dose (IIV/LAIV) annually |
| The decision to vaccinate individuals aged 18-49 years should be based on the discretion of the doctor and the choice of the patient | |
| >50 | 1 dose annually |
| Vaccination is strongly recommended for patients at high risk for influenza |
LAIV: Live, attenuated influenza vaccine, IIV: Inactivated influenza vaccine
Table 3.
Recommendations for influenza vaccination for high-risk adults
| Medical condition/indication | Influenza vaccination recommendations | Grade |
|---|---|---|
| Diabetes with or without other comorbid conditions | 1 dose annually | 2A |
| Renal diseases (CKD, end-stage renal disease on hemodialysis and peritoneal dialysis, kidney transplant recipients) | 2A | |
| Liver diseases (chronic liver disease and liver transplants) | 3A | |
| Heart diseases+ (atherosclerotic heart disease, cardiomyopathy/chronic congestive heart failure, and congenital heart disease) | 1A | |
| Patients on long-term cortisone therapy | 2A | |
| Neurological conditions^ (epilepsy, stroke, cerebral palsy, spinal injury) | UPP | |
| Hematological conditions (asplenia, sickle cell disease) | 1B | |
| People living with HIV Ø | 1B | |
| Cancer | 3A | |
| Health-care workers, working in hospital/institutional settings (doctors, nurses, paramedics) with likelihood of exposure to influenza virus. Health-care workers include | 1A | |
| All medical and paramedical personnel working in casualty/emergency department of identified hospitals treating influenza cases | ||
| All medical and paramedical personnel working in the ICU and isolation wards managing influenza patients | ||
| All personnel identified to work in screening centers that would be set up for categorization of patients during seasonal influenza outbreaks | ||
| Treating/managing high-risk patient groups | ||
| Laboratory personnel working in virological laboratories testing suspected influenza samples | ||
| Rapid-response team members identified to investigate outbreaks of influenza | ||
| Drivers and staff of vehicles/ambulances involved in transfer of influenza patients | ||
| Chronic respiratory diseases (asthma*, COPD, bronchiectasis, interstitial lung disease, and chronic smokers) | Single-dose annually favored in chronic persistent asthma; however, the decision to vaccinate should be taken as per physicians’ discretion | UPP (for asthma) |
| For all other respiratory diseases: one dose annually | 2A (except asthma) | |
| Pregnancy# LAIV: Contraindicated LAIV: Used with precaution | Single-dose annually recommended for all pregnant women | 2A |
| The vaccine can be given in any trimester of pregnancy, taking into account the seasonal strain of influenza | UPP |
*Specific robust evidence for asthmatic children>6 months- <18 years present; however, uncertainty about the effectiveness of influenza vaccination in adults (>18 years), +Influenza vaccination prevents primary cardiovascular events as well, #Influenza vaccination during pregnancy provides benefit to both the mother and infant, ^Limited data available regarding effectiveness in neurological disorders. Most data from studies evaluating vaccination rates, ØLimited data available regarding efficacy. Most data confirm immunogenicity. CKD: Chronic kidney disease, ICU: Intensive care unit, COPD: Chronic obstructive pulmonary disease, LAIV: Live, attenuated influenza vaccine, HIV: Human immunodeficiency virus
INTRODUCTION
Pneumonia and influenza are key clinical conditions encountered by clinicians and pulmonologists in India. Several evidence-based global guidelines and recommendations from numerous bodies are available for pneumococcal and influenza vaccination for the diverse adult population. However, due to differences in the disease burden, regulatory landscape, health-care infrastructure, and functioning in India, these global guidelines cannot be implemented unconditionally in the region. Hence, in the absence of country-specific structured clinical recommendations on adult respiratory vaccination, this document is a first-of-its-kind multidisciplinary effort to lay down specific evidence-based recommendations on adult pneumococcal and influenza vaccination for the country. The document further aims (1) to guide practicing clinicians and health-care professionals in the country from various specialties to make informed decisions about adult respiratory vaccination and (2) to facilitate consistent adult respiratory vaccination in the region and further help reduce the disease burden in the country.
METHODOLOGY
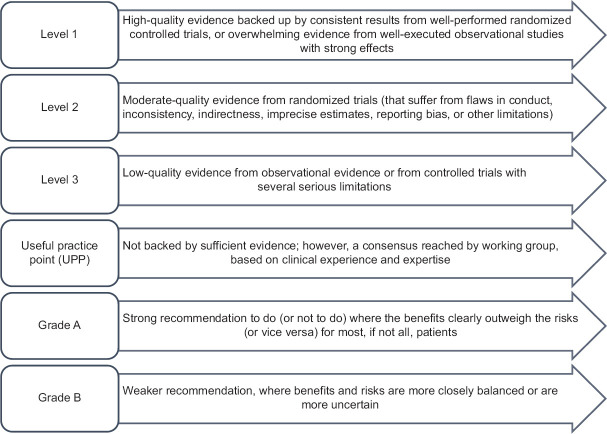
The pioneering attempt to develop easily implementable clinical recommendations for the prevention of pneumonia and influenza with vaccination in India was undertaken as a joint exercise by the Indian Chest Society and National College of Chest Physicians of India by a panel of experts at a meeting held in Kolkata on 13 January 2019. There were 24 expert members and four moderators. The expert panel members included pulmonologists, nephrologists, diabetologists, and immunologists from various parts of India. These experts critically analyzed existing literature, including randomized clinical trials, systematic reviews, and meta-analyses, as well as key global and Indian guidelines and recommendations on pneumococcal and influenza vaccination. Evidence to frame specific questions was obtained through a literature search of MEDLINE (via PubMed) and Cochrane-indexed databases on pneumococcal and influenza vaccination published between January 1995 and December 2018. The keywords for the literature search were as follows: definitions, epidemiology, India, pneumonia, influenza vaccines, trivalent influenza vaccine, quadrivalent vaccine antigenic drift, corticosteroid, statins, storage, prevention, mortality, impact, elderly, serotype, antiviral therapy, safety, immunogenicity, comorbid conditions, immunosenescence, community-acquired pneumonia, mass gatherings, antibiotics, guidelines, and recommendations. These questions on pneumococcal and influenza vaccination were formulated following discussions about several factors unique to the Indian context. Literature reviews and discussions for each disease were coordinated by group chairs and were well documented by rapporteurs. Discussions regarding the grading of evidence and recommendations were held independently in four parallel-group sessions and, thereafter, in a joint session including all the experts. A consensus-based approach was used to arrive at the final decision on clinical recommendations in the joint session. The modified grade system was utilized for categorizing the level of evidence as 1, 2, 3, or UPP [Figure 2].[1] The strength of recommendation was graded as A or B, based on the level of evidence. Grade A should be interpreted as “recommended,” while Grade B as “suggested.” All aspects related to practicality, implementation, costs, and clinical feasibility in the region at various health-care levels were duly considered while formulating the clinical recommendations.[2]
Figure 2.
Classification of level of evidence and grading of clinical recommendations[1]
EPIDEMIOLOGY
1. What is the burden of influenza globally and in India?
Influenza is a communicable respiratory viral illness infecting the nose, throat, and bronchi. It is characterized by a sudden onset of fever, cough, headache, muscle and joint pain, sore throat, and a runny nose. Most individuals recover from the symptoms within a week without requiring medical attention. However, in high-risk groups, it may cause severe illness or death.[3,4] There are four main variants for the influenza virus (A, B, C, and D); seasonal flu epidemics are caused by human influenza variants A and B.[3,5]
According to recent global estimates, influenza accounts for substantial mortality (over 600,000 deaths annually). Wide variations exist between influenza-associated mortality estimates between countries.[6,7] India is not left untouched by the condition and has been estimated to have the highest burden of mortality in connection with the infamous 1918 H1N1 pandemic, translating to about 10–20 million of the estimated global mortality of 50–100 million deaths.[8] Although there is a dearth of robust data on the disease burden in India, according to estimates from 2010 to 2017, over 100,000 cases and 8000 deaths were reported due to influenza in India. The case fatality rate in a decade-long period data analysis study was 7.5%.[9] According to Indian regional surveillance studies, the incidence of influenza hospitalizations is high as 46.8/10,000 patients. In these studies, seasonal influenza types A and B were the predominant variants of influenza.[9,10,11]
Prevention of influenza through vaccination
The influenza type A virus has historically been associated with various pandemics. The virus is divided into subtypes based on surface proteins: hemagglutinin (H) and neuraminidase (N). Various combinations of the 18 different subtypes of H and 11 different subtypes of N lead to various circulating type A viruses.[12,13]
Furthermore, the influenza virus can constantly change through the phenomena of antigenic drift and antigenic shift. Antigenic drift refers to minor genetic changes that are caused by point mutation within the antibody-binding sites of the virus, thus preventing the binding of the virus with a neutralizing antibody. By contrast, antigenic shift occurs infrequently and involves major changes in the influenza virus, resulting in new H and/or H and N proteins, thus leading to the formation of a new influenza virus subtype.[14] It is crucial to monitor these changes, as the severity of the disease is dependent on them. If the changes remain small, humans may still have partial immunity to the modified virus, thus resulting in a milder form of influenza compared with major changes, which leads to severe forms of the disease. However, if the virus undergoes antigenic shift, a possible influenza pandemic may occur against the backdrop of a lack of immunity to the mutated virus.[12,15]
2. What is the difference in the recommended influenza vaccine strains for the Northern and Southern Hemispheres, according to the World Health Organization (WHO)? Should India be included under the Southern Hemisphere for the WHO recommendation for vaccine strains?
As influenza viruses constantly change, the WHO reviews the prevalence data on circulating viruses from surveillance studies from over 100 countries twice a year (February and September). Based on these results, they propose the composition of the influenza vaccine for the Northern and Southern Hemispheres. The latest WHO-recommended composition for influenza vaccine for the two hemispheres is presented in Table 4.[16,17]
Table 4.
World Health Organization -recommended influenza vaccine composition
| Hemisphere | Composition |
|---|---|
| Northern | Quadrivalent vaccine |
| A/Brisbane/02/2018 (H1N1) pdm09-like virus | |
| A/Kansas/14/2017 (H3N2)-like virus | |
| B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) | |
| B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) | |
| Trivalent vaccine | |
| A/Michigan/45/2015 (H1N1) pdm09-like virus | |
| A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus | |
| B/Colorado/06/2017-like virus of the B/Victoria/2/87 lineage | |
| Southern | Quadrivalent vaccine |
| A/Michigan/45/2015 (H1N1) pdm09-like virus | |
| A/Switzerland/8060/2017 (H3N2)-like virus | |
| B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) | |
| B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) | |
| Trivalent vaccine | |
| A/Michigan/45/2015 (H1N1) pdm09-like virus | |
| A/Switzerland/8060/2017 (H3N2)-like virus | |
| B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) |
Geographically, India falls in the tropical region and the subtropical region in Northern Hemisphere. However, laboratory-based surveillance data from the WHO's FluNet database, created to ascertain the peak of influenza circulation in 125 countries, reveal that a 30% influenza positivity rate in December–May and 70% influenza positivity rate in June–November in India.[18,19] Evidence from a study examining the relationship between influenza positivity and the latitudes revealed that as against the typical NH pattern expected for a NH geography, Indian cities located north of the 30°N latitude and with a late monsoon season had influenza peaks in winters, while cities located south of 30°N had peak seasonality during the summer months (July–September). Hence, for the recommendation of influenza vaccine, it is essential to consider the local epidemiological data for influenza peak seasonality, rather than WHO-based hemisphere classification.[18,20,21]
3. What are the various types of vaccines available for the prevention of influenza in India and how is the nasal influenza vaccine useful in the Indian clinical scenario?
Although influenza vaccines generally have variable and only moderate efficacy (~60%), they remain a key preventive strategy for the control of the disease and its consequences.[22,23,24] At present, inactivated quadrivalent influenza vaccines are commercially available and recommended for clinical use in the Indian population.[25] High-dose trivalent IIV, adjuvanted trivalent IIV (MF59), and IIV produced using recombinant DNA technology, as well as a baculovirus expression system, are not yet available in India.[26] Another influenza vaccine indigenously developed in India is the LAIV, which is administered intranasally. Data reveal that over 2.5 million individuals have been vaccinated with LAIV with no serious adverse events or vaccine failure reported after 3 months.[27,28] Furthermore, data from a postmarketing surveillance study conducted among 7565 individuals aged 3 to 85 years revealed adverse reactions in only 1% of the study population.[28]
Although the safety of the LAIV was reassuring, there was a paucity of data on whether LAIV was as effective as contemporary IIV across various age groups. An observational study indicated that LAIV was poorly effective against influenza A (H1N1) pdm09-like viruses and was significantly less effective than IIV. However, it was effective against influenza B viruses, and the effectiveness of LAIV and IIV against influenza A (H3N2) viruses generally did not differ significantly.[29] The LAIV vaccine has been withdrawn from the Indian market and is, hence, no longer available to clinicians.[30]
Recommendations
-
For the prevention of influenza in India, two influenza vaccines are commercially available for clinical use:
- Standard-dose trivalent IIV
- Standard-dose quadrivalent IIV
Despite the availability of robust Indian safety data and the safety of LAIV (nasal vaccine), the absence of robust effectiveness evidence for LAIV compared to IIV across various age groups in India led the panel to recommend the use of IIV over LAIV. The panel further added that LAIV, if available, may be used alternately in special circumstances, based solely on the prescribing clinician's discretion.
4. Is there any preference for any specific vaccine over one another among the various available influenza vaccines?
The two clinically used influenza vaccines (trivalent and quadrivalent) vary by one influenza B strain (either Victoria or Yamagata lineage). Hence, administration of the quadrivalent vaccine is likely to provide additional protection against circulating B viruses. Results from trials conducted across various age groups, summarized in Table 5, present a strong rationale for administering quadrivalent influenza vaccines over trivalent vaccines.[31,32,33]
Table 5.
Comparison of trivalent and quadrivalent influenza vaccines
| Publication (year) | Study design and analysis | Key observations |
|---|---|---|
| Domachowske et al., 2013[31] | Phase III, double-blind, randomized, multileft study in children aged 3-17 years with stable health or chronic illness | QIV was highly immunogenic, with seroconversion rates of 91.4%, 72.3%, 70.0%, and 72.5% against A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively |
| Immunologic noninferiority of QIV versus. TIV against shared influenza A and B strains, and superiority against influenza B of QIV versus. TIVs containing an alternate-lineage B strain | QIV may offer improved protection against influenza B in children | |
| Kieninger et al. 2013[32] | Phase III, randomized, partially blind, multinational study in individuals aged ≥18 years and who were in stable health without significant pulmonary, cardiovascular, hepatic, or renal disease | QIV displayed superior immunogenicity for the alternative-lineage B strain, without impairing immune responses to shared strains |
| Reactogenicity and safety profile for QIV versus TIV | ||
| Tinocoa et al. 2013[33] | Phase III, randomized, double-blind study in individuals aged ≥18 years | QIV provided superior immunogenicity for the added B strain without affecting the antibody response to TIV strains, and without compromising safety |
| Immunogenicity, reactogenicity, and safety of QIV and TIV |
QIV: Quadrivalent influenza vaccine, TIV: Trivalent influenza vaccine
Furthermore, apart from effectiveness, the quadrivalent vaccine provides a greater reduction in influenza-related morbidity; it is also more cost-effective in developing regions such as South Africa and Vietnam compared with Australia.[34]
Recommendation
The experts recommended the use of quadrivalent IIV over trivalent IIV in developing nations such as India (Grade 2A).
5. What are the contraindications and precautions for various influenza vaccines?
Although the influenza vaccines have a good safety profile, certain precautions must be observed when using them. The key precautions and contraindication for the widely used influenza vaccine in India, viz., the IIV, are listed in Table 6.[35,36,37]
Table 6.
Contraindications and precautions for inactivated influenza vaccine
| Inactivated influenza vaccine (IIV) | |
|---|---|
| Contraindications | Precautions |
| Previous severe allergic reaction to influenza vaccine, regardless of the component suspected of being responsible for the reaction, is a contraindication for future use of the vaccine. | Moderate or severe acute illness with or without fever History of GBS within 6 weeks of receipt of influenza vaccine |
GBS: Guillain-Barré syndrome
Recommendation
The experts accepted the established precautions and contraindications for various vaccines presented in Table 6.
6. What are the recommendations for influenza vaccination for individuals with egg allergy and previous history of Guillain–Barré syndrome (GBS)?
Influenza viruses are propagated in eggs; hence, the chance of incorporation of antigen in patients previously exposed remains high. Hence, a history of egg allergy needs to be considered when a patient is administered IIV. There are however some recent data to suggest that patients with egg allergy can safely receive IIV.[38,39]
Furthermore, according to the Advisory Committee on Immunization Practices (ACIP), the occurrence of GBS is considered a precaution for future influenza vaccination. If an individual has experienced GBS within 6 weeks of previous influenza vaccination, they should not be vaccinated.[40]
Recommendations
-
Individuals with a history of egg allergy and who have:
- Experienced only urticaria (hives) after exposure to egg, can receive an age-appropriate influenza vaccine (IIV or LAIV) (Grade UPP)
- Experience reactions to egg involving symptoms other than urticaria (hives), such as angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or another emergency medical intervention, can receive an age-appropriate influenza vaccine (IIV or LAIV) (Grade UPP)
- A previous history of a severe allergic reaction to the vaccine, regardless of the component of the vaccine responsible, is contraindicated to the use of IIV (Grade UPP).
Consistent with the existing evidence, the experts recommended not to observe any postvaccination observation period for individuals who are egg-allergic (Grade UPP)
The experts recommended against the use of influenza vaccination in individuals who are not at higher risk for severe influenza complications and known to have experienced GBS within 6 weeks of previous influenza vaccination (Grade-UPP).
7. What is the recommendation for influenza vaccination for patients with multiple sclerosis?
The seasonal influenza vaccine has been studied extensively in MS patients; evidence suggests it to be safe, regardless of the disease-modifying therapy patients are receiving.[41] Furthermore, evidence also shows that influenza vaccination does not have a detrimental effect on disease progression in MS.[42] However, according to ACIP recommendations, MS patients should be administered inactivated vaccine and avoid LAIV.[40]
Recommendation
The experts recommended a SD of IIV and avoidance of LAIV for MS patients (Grade UPP).
8. What is the recommendation for influenza vaccination for patients on antiviral therapy?
Administration of IIV to individuals receiving influenza antiviral medications for treatment or chemoprophylaxis is acceptable. However, antiviral medications have the propensity to reduce the effectiveness of LAIV if given within 48 h before to 14 days after LAIV.[36]
Recommendation
The experts recommended influenza vaccination with IIV for individuals receiving antiviral medications either for treatment or chemoprophylaxis (Grade UPP).
9. What is the recommendation for influenza vaccination for patients on corticosteroid therapy?
Patients with chronic pulmonary diseases who regularly receive inhaled or/and systemic corticosteroids can generate an antibody response against the influenza virus. In a prospective study conducted among patients with chronic respiratory diseases who were on/off corticosteroid therapy, the seroprotection for hepatitis B surface antibody (HB) was found to be significantly higher in patients not on corticosteroid therapy compared to patients on corticosteroid therapy. Furthermore, no systemic reactions were observed, and the study recommended that long-term oral/inhaled corticosteroid therapy should not preclude the administration of influenza vaccine in patients with chronic pulmonary diseases, who are considered to be at high risk for contracting the disease.[43,44]
Recommendation
The panel acknowledged the antibody response against the influenza vaccine by corticosteroid therapy, but suggested that long-term corticosteroid therapy in an individual should not preclude vaccine use in patients with chronic pulmonary disease (Grade 2A).
10. What is the recommendation for influenza vaccination for patients with concomitant statin therapy?
Statins are known to have anti-inflammatory effects involving various components of the innate and adaptive immune systems. They decrease levels of inflammatory markers, affecting antibody class switching and antibody expression in an individual.[45] Evidence generated from a population-based study conducted among patients aged ≥45 years suggests that the use of statins is associated with a reduction in vaccine effectiveness against influenza A (H3N2). The vaccine effectiveness against influenza A (H3N2) was 45% (95% confidence interval [CI], 27% to 59%) among statin nonusers compared to −21% (95% CI, −84% to 20%) among statin users.[46] Furthermore, results from a retrospective cohort study conducted among patients aged >65 years on statin therapy who received high/standard-dose influenza vaccine revealed that the use of statins around the time of vaccination does not substantially affect the risk of influenza-related medical encounters among older adults.[47]
Recommendation
The experts acknowledged that statins have the propensity to reduce the effectiveness of the influenza vaccine, but nevertheless suggested the use of influenza vaccination in such patients (Grade 3A).
11. What is the coadministration schedule of influenza vaccines with respect to other recommended vaccines?
Data regarding simultaneous or sequential administration of the influenza vaccine and other combinations of vaccines are limited. However, evidence reveals that the IIV does not interfere with the immune response to other inactivated vaccines or to live virus vaccines. The IIV was found to be well-tolerated and demonstrated no immunogenicity changes in adults of either sex aged >50 years when administered at different anatomical sites with the herpes zoster vaccine, conjugated and unconjugated pneumococcal vaccines.[48,49,50,51,52] However, the ACIP guidelines recommend administration of a live vaccine (LAIV); at least 4 weeks should elapse before another live vaccine is administered.[40]
Recommendations
In line with the evidence, the experts accepted and recommended that IIV can be concomitantly or sequentially administered with other inactivated vaccines or with live vaccines (Grade 1A)
For LAIVs, the experts recommended maintaining at least 4 weeks' gap between the administration of LAIV and another live vaccine (Grade UPP)
LAIV does not interfere with the immune response of MMR or varicella vaccines; hence, the experts recommended that they can be administered at the same visit (Grade 1A). However, the experts suggested that injectable vaccines that are given concomitantly with the influenza vaccine should be administered at separate anatomical sites (Grade UPP).
12. What are the recommended storage and handling specifications for various influenza vaccines?
According to the prescribing instruction and recommendations, the influenza vaccine should be refrigerated at 2°C–8°C (36°F–46°F) in a stand-alone or combination refrigerator and should not be frozen. The product should be discarded if found frozen. Furthermore, the vaccine should never be stored in the door of the refrigerator or the door of the freezer. This would expose the vaccine to wide temperature variations. The refrigerator should be equipped with a certified and calibrated thermometer. The temperature in the refrigerator should be checked at least once each day, and the control should be adjusted slightly if the thermometer indicates that the temperature is close to either of the two extremes (35° and 46°F). The electrical supply to the refrigerator should be safeguarded.[53]
For transportation of the vaccine, the temperature inside the packed container, along with the date, time, and initials, should be recorded. Upon arrival at the facility, the vaccine should be immediately transferred to a refrigerator that maintains the recommended temperature range; the temperature, time, and initials should be recorded. If the vaccine must be kept in a portable storage unit, monitor the temperature hourly.[53]
Recommendations
-
The expert-recommended storage of the influenza vaccine is as follows:
- Only in a standout refrigerator, within a temperature range of 2°C–8°C (average temperature of 5°C) (Grade UPP)
- The vaccine should never be frozen or stored in the door of the refrigerator (Grade UPP).
Household refrigerators are not recommended for storing the vaccine, due to their lack of temperature uniformity and due to the risk of contamination and power outages (Grade UPP)
For transportation of influenza vaccines, the experts recommended the use of cold chains with immediate transfer of vaccines to a refrigerator with a uniform temperature range upon arrival. For storage of influenza vaccines in a portable storage unit, the temperature should be well maintained and recorded (Grade UPP).
13. What is the recommended dose and frequency of influenza vaccination in India?
Evidence reveals a significantly increased level of antibody response with a high dose (HD) of influenza vaccine compared to the SD of vaccine. Further, there was no proportionate increase in the incidence and severity of clinically relevant adverse reactions with HD versus SD.[54,55,56] For elderly patients (>65 years), in a single-blind comparative trial, the HD of influenza vaccine was associated with a significantly lower incidence of respiratory-related hospital admissions compared to standard-dose influenza vaccine (0.185/1000 resident-days or 3.4% over 6 months versus 0.211/1000 resident-days or 3.9% over 6 months).[56]
As regards the frequency of vaccination, the ACIP recommends a single dose of influenza vaccine annually soon after the vaccine becomes available. As the influenza virus undergoes antigenic shifts, and vaccine-specific antibodies decline with time, annual revaccination is required.[57]
Annual vaccination is also associated with a reduction in mortality in older adults who have been vaccinated previously, but not in those who received the vaccine for the first time. Evidence generated from a population-based study revealed an annual reduction in mortality risk of 10%; revaccination reduced the annual mortality risk by 15%.[58]
Recommendations
The experts recommended a single HD of IIV, when available, for Indian clinical practice (Grade 1A)
In view of the dearth of evidence comparing single-dose annual influenza vaccination and biannual influenza vaccination, the experts recommended annual influenza vaccination (Grade UPP)
14. What is the ideal timing for influenza vaccination in India?
As different parts of the country have variations in peak influenza seasonality, a uniform pattern for administering the influenza vaccine cannot be maintained. The nation should be subdivided into regions based on latitude, as well as disease seasonality.[9] As discussed earlier, cities with >30°N of latitude and with a late monsoon season show influenza peaks in winters, while cities with <30°N show influenza peaks in July–September. Hence, for the appropriate timing for the influenza vaccine, it is essential to consider the local epidemiological data for influenza peak seasonality.[18,20,21]
Recommendation
The experts recommended influenza vaccination from September to October for cities with temperate seasonality and April–May for cities with the peak monsoon season in July–September [Table 7] (Grade UPP).
Table 7.
Timing for influenza vaccination in India
| Proposed vaccination timing | Cites |
|---|---|
| April-May | Delhi, Lucknow, Kolkata, Dibrugarh, Pune |
| September-October | Srinagar, Chennai, Vellore |
15. Are individual regional protocols for influenza vaccination required in India?
Considering India's large landmass, varying geography, and variable influenza seasonality, there is a need to develop region-specific influenza vaccination schedules. These regional protocols for individual states (based on surveillance studies) will lead to a greater emphasis on the best period for influenza vaccination administration. Regional protocols can also emphasize the need for influenza vaccination in the local population in the local language, thereby increasing the outreach of the preventive strategy.
Recommendation
The panel acknowledged the need for regional influenza vaccination and unanimously agreed on the need for in-depth national surveillance data to observe influenza seasonality in various Indian states. These data will further help formulate peak-seasonality-based individual recommendations for different regions of India (Grade UPP)
16. What are the recommendations for influenza vaccination for adults in India?
The experts acknowledged the importance of the cost-effectiveness of influenza vaccination in a developing nation such as India, specifically for adults aged >50 years. The recommendations specific to the Indian population proposed by the panel are presented in Table 8 (Grade UPP).
Table 8.
Influenza vaccination recommendations for Indian adults
| Age group (years) | Influenza vaccination recommendations |
|---|---|
| 18-49 | 1 dose (IIV/LAIV) annually |
| The choice of vaccine to be administered is based on the discretion of the doctor and patient while vaccinating individuals aged 18-49 years | |
| >50 | 1 dose annually |
| Vaccination is strongly recommended for patients at high risk for influenza |
LAIV: Live, attenuated influenza vaccine, IIV: Inactivated influenza vaccine
17. What are the vaccination recommendations for adults at high risk of influenza in India?
Based on the evidence, the experts recommended routine influenza vaccination for Indians with specific medical conditions and indications that predispose to a high risk of complications of influenza. The recommendations for influenza vaccination for high-risk adult groups are presented in Table 9.[59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]
Table 9.
Influenza vaccination recommendation in Indian adults categorized as high-risk group
| Medical condition/indication | Influenza vaccination recommendations | Grade |
|---|---|---|
| Diabetes with or without other comorbid conditions | 1 dose annually | 2A |
| Renal diseases (CKD, end-stage renal disease on hemodialysis and peritoneal dialysis, kidney transplant recipients) | 2A | |
| Liver diseases (chronic liver disease and liver transplants) | 3A | |
| Heart diseases+ (atherosclerotic heart disease, cardiomyopathy/chronic congestive heart failure, and congenital heart disease) | 1A | |
| Patients on long-term cortisone therapy | 2A | |
| Neurological conditions^ (epilepsy, stroke, cerebral palsy, spinal injury) | UPP | |
| Hematological conditions (Asplenia, sickle cell disease) | 1B | |
| People living with HIVØ | 1B | |
| Cancer | 3A | |
| Health-care workers, working in hospitals/institutional settings (doctors, nurses, paramedics) with likelihood of exposure to influenza virus. Health-care workers include | 1A | |
| All medical and paramedical personnel working in casualty/emergency department of identified hospitals treating influenza cases | ||
| All medical and paramedical personnel working in the ICU and isolation wards managing influenza patients | ||
| All personnel identified to work in screening centers that would be set up for categorization of patients during seasonal influenza outbreaks | ||
| All personnel treating/managing high-risk patient groups | ||
| Laboratory personnel working in virological laboratories testing suspected influenza samples | ||
| Rapid-response team members identified to investigate outbreaks of influenza | ||
| Drivers and staff of vehicles/ambulances involved in transfer of influenza patients | ||
| Chronic respiratory diseases (asthma*, COPD, bronchiectasis, interstitial lung disease, and chronic smokers) | 1 dose annual vaccination favored in chronic persistent asthma; however, the decision to vaccinate should be taken as per the physician’s discretion | UPP (for asthma) |
| For all other respiratory diseases: 1 dose annually | 2A (except asthma) | |
| Pregnancy# LAIV: Contraindicated; LAIV: Used with precaution | Vaccination recommended for all pregnant women | 2A |
| The vaccine can be given in any trimester of pregnancy with due consideration to the seasonal strain of influenza | UPP |
*Specific robust evidence for asthmatic children >6 months–<18 years present; however, uncertainty about the effectiveness of influenza vaccination in adults (>18 years), +Influenza vaccination prevents primary cardiovascular events as well, #Influenza vaccination during pregnancy provides benefits to both the mother and infant, ^Limited data available regarding effectiveness in neurological disorders. Most data from studies evaluating vaccination rates, ØLimited data available regarding efficacy. Most data confirm immunogenicity. CKD: Chronic kidney disease, ICU: Intensive care unit, COPD: Chronic obstructive pulmonary disease, LAIV: Live, attenuated influenza vaccine, HIV: Human immunodeficiency virus
18. What is the recommendation for influenza vaccination for travelers, Hajj pilgrims, and individuals attending mass gatherings?
Seasonal influenza is one of the most common preventable air-borne diseases in travelers. The risk of exposure to the disease during travel depends on the time of year and the destination of travel (Northern/Southern Hemisphere).[82] Each hemisphere has its unique peak influenza activity time. In the Northern Hemisphere, the influenza season begins in October and lasts until April or May. In the Southern Hemisphere, influenza activity is at its peak between April and September. Tropical regions have influenza activity throughout the year.[82] Evidence generated based on analysis of decade-long period surveillance data gathered from over 30,000 ill-returned travelers reveals that tourists/travelers visiting Southeast and East Asia have a sevenfold higher risk for influenza compared to travelers visiting other destinations.[83] The severity and outcomes of the disease are also dependent on patient-related factors, such as age, immune, and health status.
Hence, to prevent travelers from contracting influenza, influenza vaccination with strains of the hemisphere/destination being visited is recommended at least 2 weeks before travel. The recommendation becomes all the more crucial for adults >50 years traveling to tropical regions or the Northern or Southern Hemisphere during the winter months; for those traveling on a cruise ship in large groups, as well as for those attending mass gathering events such as Hajj and Umrah.[82,83,84,85,86]
Religious festivals involve huge participation from pilgrims coming from around the globe and can potentially lead to the transmission of infectious diseases among pilgrims. The mass gathering of pilgrims from various regions of the world has the propensity to compromise the health system of the hosting country. More than 2 million pilgrims participate in Hajj or the Umrah each year.[87] Data generated over the years, from the assessment of Hajj-returned pilgrims, show respiratory viral infections to be the most prevalent infection among Hajj pilgrims.[87] With respect to India, a study conducted among 500 Hajj pilgrims revealed that the influenza virus (A and B) was the most common respiratory viral respiratory infection.[87]
Hence, both Indian and host-country (Saudi Arabia) health-care bodies recommend influenza vaccination for all pilgrims and health-care workers working in the Hajj region before the start of the rituals.[84,85] In a meta-analysis by Alqahtani et al. including six prospective studies, influenza vaccination showed significantly protective action against laboratory-confirmed influenza among Hajj pilgrims.[88] Although studies on other religious congregations such as the Kumbh are lacking, recommendation of influenza vaccination and data from Hajj pilgrims might be considered for all very large religious gatherings.
Recommendations
Influenza vaccination is recommended for individuals (irrespective of their vaccination status) traveling to parts of the world where influenza activity is ongoing. The administered influenza vaccination should consist of strains that are currently circulating at the travel destination. The vaccine is recommended to be administered at least 2 weeks before the scheduled departure (Grade UPP)
The experts recommended influenza vaccination for Hajj pilgrims (Grade 2A) and individuals attending mass gatherings, irrespective of the influenza season (at home or the destination) (Grade UPP).
19. Is it justifiable to classify individuals into various priority risk groups for influenza vaccination in India?
In resource-limited settings, such as India, a risk-group-based categorization of patients is justifiable. The Ministry of Health and Family Welfare, India, provides such a classification for adults.[25]
Recommendations
The experts accepted and agreed upon the categorization of high-risk influenza groups laid down by the Ministry of Health and Family Welfare, India. They further suggested the inclusion of veterinary, poultry workers, and military personnel in the high-risk influenza group (Grade UPP)
High-risk influenza groups, as per the Ministry of Health and Family Welfare, India, are mentioned in Table 10.
Table 10.
List of the high-risk influenza groups as per the Ministry of Health and Family Welfare, India
| Health-care workers working in hospitals/institutional settings (doctors, nurses, paramedics) with likelihood of exposure to influenza virus. These include |
| All medical and paramedical personnel working in casualty/emergency department of identified hospitals treating influenza cases |
| All medical and paramedical personnel working in ICU and isolation wards managing influenza patients |
| All personnel identified to work in screening centers that would be set up for categorization of patients during seasonal influenza outbreaks |
| Those treating/managing high-risk groups |
| Laboratory personnel working in virological laboratories testing suspected influenza samples |
| Rapid-response team members identified to investigate outbreaks of influenza |
| Drivers and staff of vehicles/ambulances involved in transfer of influenza patients |
| Vaccination is recommended for |
| Pregnant women, irrespective of the duration of pregnancy |
| People with chronic illnesses, such as chronic obstructive pulmonary disease, bronchial asthma, heart disease, liver disease, kidney disease, blood disorders, diabetes, cancer; and for those who are immunocompromised |
| In children with chronic diseases such as asthma; neurodevelopmental conditions such as cerebral palsy, epilepsy stroke, mentally challenged, etc.; heart disease such as CHD, CHF; blood disorders such as sickle cell disease; diabetes, metabolic disorders; all immunocompromised children; malignancy receiving immuno-suppressive therapy; kidney disorder; and liver disorder |
| Vaccination is desirable for: |
| Elderly individuals (≥65 years of age) |
| Children aged 6 months to 8 years of age |
ICU: Intensive care unit, CHD: Coronary heart disease, CHF: Congestive heart failure
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge BioQuest Solutions in compiling the literature, data analysis, and finalizing the article.
REFERENCES
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group.GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–26. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt G, Rennie D, Meade MO, Cook DJ. Users' Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 2nd ed. New York: McGraw Hill; 2008. [Google Scholar]
- 3.WHO. Influenza (Seasonal) Ask The Expert. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwwhoint/en/news-room/fact-sheets/detail/influenza-(seasonal)
- 4.Centers for Disease Control and Prevention. Influenza (Flu) Key Facts About Influenza (Flu) [[Last accessed on 2019 Dec 03]]. Available from: https://wwwcdcgov/flu/about/keyfactshtm .
- 5.Centers for Disease Control and Prevention. Influenza (Flu) Understanding Influenza Viruses. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwcdcgov/flu/about/viruses .
- 6.WHO. Up to 650 000 People die of Respiratory Diseases Linked to Seasonal Flu Each Year. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwwhoint/news-room/detail/14-12-2017-up-to-650-000-people-die-of-respiratory-dise ases-linked-to-seasonal-flu-each-year .
- 7.Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018;391:1285–300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kant L, Guleria R. Pandemic Flu, 1918: After hundred years, India is as vulnerable. Indian J Med Res. 2018;147:221–4. doi: 10.4103/ijmr.IJMR_407_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee P, Seth B, Biswas T, Bera K. Burden of H1N1 Influenza in India (2010-2017): Identifying Hotspots and Policy Directions. ATS International Conference Abstracts. In: C41. Health Services Research in Pulmonary Disease. Am J Respir Crit Care Med. 2018:pA4948. [Google Scholar]
- 10.Chadha MS, Hirve S, Dawood FS, Lele P, Deoshatwar A, Sambhudas S, et al. Burden of seasonal and pandemic influenza-associated hospitalization during and after 2009 A (H1N1) pdm09 pandemic in a rural community in India. PLoS One. 2013;8:e55918. doi: 10.1371/journal.pone.0055918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broor S, Krishnan A, Roy DS, Dhakad S, Kaushik S, Mir MA, et al. Dynamic patterns of circulating seasonal and pandemic A (H1N1) pdm09 influenza viruses from 2007-2010 in and around Delhi, India. PLoS One. 2012;7:e29129. doi: 10.1371/journal.pone.0029129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: A structured literature review. Am J Public Health. 2013;103:e43–51. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Types of Influenza Viruses. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwcdcgov/flu/about/viruses/typeshtm .
- 14.Kim H, Webster RG, Webby RJ. Influenza virus: Dealing with a drifting and shifting pathogen. Viral Immunol. 2018;31:174–83. doi: 10.1089/vim.2017.0141. [DOI] [PubMed] [Google Scholar]
- 15.Lewis NS, Daly JM, Russell CA, Horton DL, Skepner E, Bryant NA, et al. Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. J Virol. 2011;85:12742–9. doi: 10.1128/JVI.05319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Recommended Composition of Influenza Virus Vaccines for use in the 2019 Southern Hemisphere Influenza Season. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwwhoint/influenza/vaccines/virus/recommendations/201809_recommendationpdfua=1 .
- 17.WHO. Recommended Composition of Influenza Virus Vaccines for use in the 2019-2020 Northern Hemisphere Influenza Season. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwwhoint/influenza/vaccines/virus/recomme ndations/201902_recommendationpdfua=1 .
- 18.Alonso WJ, Yu C, Viboud C, Richard SA, Schuck-Paim C, Simonsen L, et al. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep. 2015;5:17214. doi: 10.1038/srep17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha S, Chadha M, Al Mamun A, Rahman M, Sturm-Ramirez K, Chittaganpitch M, et al. Influenza seasonality and vaccination timing in tropical and subtropical areas of Southern and South-Eastern Asia. Bull World Health Organ. 2014;92:318–30. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chadha MS, Potdar VA, Saha S, Koul PA, Broor S, Dar L, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koul PA, Broor S, Saha S, Barnes J, Smith C, Shaw M, et al. Differences in influenza seasonality by latitude, Northern India. Emerg Infect Dis. 2014;20:1723–6. doi: 10.3201/eid2010.140431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 23.Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 24.Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seasonal Influenza: Guidelines for Vaccination with Influenza Vaccine Ministry of Health and Family Welfare. [[Last accessed on 2019 Dec 03]]. Available from: https://mohfwgovin/sites/default/files/Guidelines%20for%20Vaccination%20w ith%20Influenza%20Vaccinepdf .
- 26.Patel SS, Bizjajeva S, Heijnen E, Oberye J. MF59-adjuvanted seasonal trivalent inactivated influenza vaccine: Safety and immunogenicity in young children at risk of influenza complications. Int J Infect Dis. 2019;85S:S18–25. doi: 10.1016/j.ijid.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Dhere R, Yeolekar L, Kulkarni P, Menon R, Vaidya V, Ganguly M, et al. A pandemic influenza vaccine in India: From strain to sale within 12 months. Vaccine. 2011;29(Suppl 1):A16–21. doi: 10.1016/j.vaccine.2011.04.119. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni PS, Raut SK, Dhere RM. A post-marketing surveillance study of a human live-virus pandemic influenza A (H1N1) vaccine (Nasovac ®) in India. Hum Vaccin Immunother. 2013;9:122–4. doi: 10.4161/hv.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2018-19 influenza season. MMWR Recomm Rep. 2018;67:1–20. doi: 10.15585/mmwr.rr6703a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vashishtha VM, Kalra A, Choudhury P. Influenza vaccination in India: Position paper of Indian Academy of Pediatrics, 2013. Indian Pediatr. 2013;50:867–74. doi: 10.1007/s13312-013-0230-x. [DOI] [PubMed] [Google Scholar]
- 31.Domachowske JB, Pankow-Culot H, Bautista M, Feng Y, Claeys C, Peeters M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis. 2013;207:1878–87. doi: 10.1093/infdis/jit091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: A phase III, randomized trial in adults aged≥18 years. BMC Infect Dis. 2013;13:343. doi: 10.1186/1471-2334-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinocoa JC, Pavia-Ruz N, Cruz-Valdez A, Doniz CA, Chandrasekaran V, Dewe W, et al. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged≥18 years: A phase III, randomized trial. Vaccine. 2014;32:1480–7. doi: 10.1016/j.vaccine.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 34.de Boer PT, Kelso JK, Halder N, Nguyen TP, Moyes J, Cohen C, et al. The cost-effectiveness of trivalent and quadrivalent influenza vaccination in communities in South Africa, Vietnam and Australia. Vaccine. 2018;36:997–1007. doi: 10.1016/j.vaccine.2017.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Influenza Vaccine STN BL 125254. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwfdagov/downloads/biologicsbloodvaccines/vaccines/approvedprod ucts/ucm263239pdf .
- 36.FDA. Flumist. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwfdagov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm294307pdf .
- 37.FDA. Quadrivalent Influenza Vaccine BLA STN 125285. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwfdagov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM619551pdf .
- 38.Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ. 2009;339:b3680. doi: 10.1136/bmj.b3680. [DOI] [PubMed] [Google Scholar]
- 39.Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M Behalf of the SNIFFLE-2 Study Investigators. Safety of LAIV in young people with egg allergy: Multicenter prospective cohort study. BMJ. 2015;351:h6291. doi: 10.1136/bmj.h6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2019-20 influenza season. MMWR Recomm Rep. 2019;68:1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: A systematic review. J Neurol. 2017;264:1035–50. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 42.National Multiple Sclerosis Society. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwnationalmssocietyorg/Living-Well-With-MS/Diet-Exercise-Healthy-Behaviors/Vaccinations .
- 43.Inoue S, Shibata Y, Takabatake N, Igarashi A, Abe S, Kubota I. Influence of corticosteroid therapy on the serum antibody response to influenza vaccine in elderly patients with chronic pulmonary diseases. EXCLI J. 2013;12:760–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Kubiet MA, Gonzalez-Rothi RJ, Cottey R, Bender BS. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. Chest. 1996;110:367–70. doi: 10.1378/chest.110.2.367. [DOI] [PubMed] [Google Scholar]
- 45.Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents? Circulation. 2004;109:II18–26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- 46.McLean HQ, Chow BD, VanWormer JJ, King JP, Belongia EA. Effect of Statin use on influenza vaccine effectiveness. J Infect Dis. 2016;214:1150–8. doi: 10.1093/infdis/jiw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izurieta HS, Chillarige Y, Kelman JA, Forshee R, Qiang Y, Wernecke M, et al. Statin use and risks of influenza-related outcomes among older adults receiving standard-dose or high-dose influenza vaccines through medicare during 2010-2015. Clin Infect Dis. 2018;67:378–87. doi: 10.1093/cid/ciy100. [DOI] [PubMed] [Google Scholar]
- 48.Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ. Comparison of immunogenicity and safety of an influenza vaccine administered concomitantly with a 13-valent pneumococcal conjugate vaccine or 23-valent polysaccharide pneumococcal vaccine in the elderly. Clin Exp Vaccine Res. 2017;6:38–44. doi: 10.7774/cevr.2017.6.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frenck RW, Jr, Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19:1296–303. doi: 10.1128/CVI.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ofori-Anyinam O, Leroux-Roels G, Drame M, Aerssens A, Maes C, Amanullah A, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine co-administered with a 23-valent pneumococcal polysaccharide vaccine versus separate administration, in adults≥50 years of age: Results from a phase III, randomized, non-inferiority trial. Vaccine. 2017;35:6321–8. doi: 10.1016/j.vaccine.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Kerzner B, Murray AV, Cheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc. 2007;55:1499–507. doi: 10.1111/j.1532-5415.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 52.Levin MJ, Buchwald UK, Gardner J, Martin J, Stek JE, Brown E, et al. Immunogenicity and safety of zoster vaccine live administered with quadrivalent influenza virus vaccine. Vaccine. 2018;36:179–85. doi: 10.1016/j.vaccine.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. Vaccine Storage and Handling. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwcdcgov/vaccines/pubs/pinkbook/downloads/vac-storagepdf .
- 54.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 55.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 56.Gravenstein S, Davidson HE, Taljaard M, Ogarek J, Gozalo P, Han L, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: A cluster-randomised trial. Lancet Respir Med. 2017;5:738–46. doi: 10.1016/S2213-2600(17)30235-7. [DOI] [PubMed] [Google Scholar]
- 57.Voordouw AC, Sturkenboom MC, Dieleman JP, Stijnen T, Smith DJ, van der Lei J, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA. 2004;292:2089–95. doi: 10.1001/jama.292.17.2089. [DOI] [PubMed] [Google Scholar]
- 58.Young B, Sadarangani S, Yew HS, Yung CF, Barr I, Connolly J, et al. Semiannual Versus Annual Influenza Vaccination in Older Adults in the Tropics: An Observer-blind, Active-comparator-controlled, Randomized Superiority Trial. Clin Infect Dis. 2019;69:121–9. doi: 10.1093/cid/ciy836. [DOI] [PubMed] [Google Scholar]
- 59.Saxén H, Virtanen M. Randomized, placebo-controlled double blind study on the efficacy of influenza immunization on absenteeism of health care workers. Pediatr Infect Dis J. 1999;18:779–83. doi: 10.1097/00006454-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Imai C, Toizumi M, Hall L, Lambert S, Halton K, Merollini K. A systematic review and meta-analysis of the direct epidemiological and economic effects of seasonal influenza vaccination on healthcare workers. PLoS One. 2018;13:e0198685. doi: 10.1371/journal.pone.0198685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Restivo V, Costantino C, Bono S, Maniglia M, Marchese V, Ventura G, et al. Influenza vaccine effectiveness among high-risk groups: A systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother. 2018;14:724–35. doi: 10.1080/21645515.2017.1321722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilde JA, McMillan JA, Serwint J, Butta J, O'Riordan MA, Steinhoff MC. Effectiveness of influenza vaccine in health care professionals: A randomized trial. JAMA. 1999;281:908–13. doi: 10.1001/jama.281.10.908. [DOI] [PubMed] [Google Scholar]
- 63.Remschmidt C, Wichmann O, Harder T. Vaccines for the prevention of seasonal influenza in patients with diabetes: Systematic review and meta-analysis. BMC Med. 2015;13:53. doi: 10.1186/s12916-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goeijenbier M, van Sloten TT, Slobbe L, Mathieu C, van Genderen P, Beyer WE, et al. Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine. 2017;35:5095–101. doi: 10.1016/j.vaccine.2017.07.095. [DOI] [PubMed] [Google Scholar]
- 65.Seo YB, Baek JH, Lee J, Song JY, Lee JS, Cheong HJ, et al. Long-term immunogenicity and safety of a conventional influenza vaccine in patients with type 2 diabetes. Clin Vaccine Immunol. 2015;22:1160–5. doi: 10.1128/CVI.00288-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scharpé J, Peetermans WE, Vanwalleghem J, Maes B, Bammens B, Claes K, et al. Immunogenicity of a standard trivalent influenza vaccine in patients on long-term hemodialysis: An open-label trial. Am J Kidney Dis. 2009;54:77–85. doi: 10.1053/j.ajkd.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 67.Bekkat-Berkani R, Wilkinson T, Buchy P, Dos Santos G, Stefanidis D, Devaster JM, et al. Seasonal influenza vaccination in patients with COPD: A systematic literature review. BMC Pulm Med. 2017;17:79. doi: 10.1186/s12890-017-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopsaftis Z, Wood-Baker R, Poole P. Influenza vaccine for chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2018;6:CD002733. doi: 10.1002/14651858.CD002733.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Menon B, Gurnani M, Aggarwal B. Comparison of outpatient visits and hospitalisations, in patients with chronic obstructive pulmonary disease, before and after influenza vaccination. Int J Clin Pract. 2008;62:593–8. doi: 10.1111/j.1742-1241.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- 70.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: A randomized controlled study. Chest. 2004;125:2011–20. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 71.Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. JAMA. 2013;310:1711–20. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 72.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions: The FLU Vaccination Acute Coronary Syndromes (FLUVACS) Study. Circulation. 2002;105:2143–7. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 73.Ciszewski A, Bilinska ZT, Brydak LB, Kepka C, Kruk M, Romanowska M, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–8. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 74.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–5. doi: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 75.Duchini A, Hendry RM, Nyberg LM, Viernes ME, Pockros PJ. Immune response to influenza vaccine in adult liver transplant recipients. Liver Transpl. 2001;7:311–3. doi: 10.1053/jlts.2001.23010. [DOI] [PubMed] [Google Scholar]
- 76.Nunes MC, Aqil AR, Omer SB, Madhi SA. The Effects of Influenza Vaccination during Pregnancy on Birth Outcomes: A Systematic Review and Meta-Analysis. Am J Perinatol. 2016;33:1104–14. doi: 10.1055/s-0036-1586101. [DOI] [PubMed] [Google Scholar]
- 77.Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: A systematic review and meta-analysis. Clin Infect Dis. 2015;60:e11–9. doi: 10.1093/cid/ciu915. [DOI] [PubMed] [Google Scholar]
- 78.Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371:918–31. doi: 10.1056/NEJMoa1401480. [DOI] [PubMed] [Google Scholar]
- 79.Bitterman R, Eliakim-Raz N, Vinograd I, Zalmanovici Trestioreanu A, Leibovici L, Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018;2:CD008983. doi: 10.1002/14651858.CD008983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ambati A, Boas LS, Ljungman P, Testa L, de Oliveira JF, Aoun M, et al. Evaluation of pretransplant influenza vaccination in hematopoietic SCT: A randomized prospective study. Bone Marrow Transplant. 2015;50:858–64. doi: 10.1038/bmt.2015.47. [DOI] [PubMed] [Google Scholar]
- 81.Vasileiou E, Sheikh A, Butler C, El Ferkh K, von Wissmann B, McMenamin J, et al. Effectiveness of influenza vaccines in asthma: A systematic review and meta-analysis. Clin Infect Dis. 2017;65:1388–95. doi: 10.1093/cid/cix524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention. Influenza Prevention: Information for Travelers. [[Last accessed on 2019 Dec 03]]. Available from: https://wwwcdcgov/flu/travelers/travelersfactshtm .
- 83.Boggild AK, Castelli F, Gautret P, Torresi J, von Sonnenburg F, Barnett ED, et al. Latitudinal patterns of travel among returned travelers with influenza: Results from the GeoSentinel Surveillance Network, 1997-2007. J Travel Med. 2012;19:4–8. doi: 10.1111/j.1708-8305.2011.00579.x. [DOI] [PubMed] [Google Scholar]
- 84.The Ministry of Health. Saudi Arabia: Health Requirements and Recommendations for Travelers to Saudi Arabia for Hajj and Umrah-2018/1439H; [[Last accessed on 2019 Dec 03]]. Available from: https://wwwmohgovsa/en/hajj/pages/healthregulationsaspx . [Google Scholar]
- 85.Health Requirement for pilgrims of Haj 1439(H)-2018. [[Last accessed on 2019 Dec 03]]. Available from: http://hajcommitteegovin/Files/Circu lar/2018/circular_2018_31pdf .
- 86.Koul PA, Mir H, Saha S, Chadha MS, Potdar V, Widdowson MA, et al. Respiratory viruses in returning Hajj and Umrah pilgrims with acute respiratory illness in 2014-2015. Indian J Med Res. 2018;148:329–33. doi: 10.4103/ijmr.IJMR_890_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. Influenza a common viral infection among Hajj pilgrims: Time for routine surveillance and vaccination. J Travel Med. 2004;11:82–6. doi: 10.2310/7060.2004.17027. [DOI] [PubMed] [Google Scholar]
- 88.Alqahtani AS, Rashid H, Heywood AE. Vaccinations against respiratory tract infections at Hajj. Clin Microbiol Infect. 2015;21:115–27. doi: 10.1016/j.cmi.2014.11.026. [DOI] [PubMed] [Google Scholar]