Figure 1.
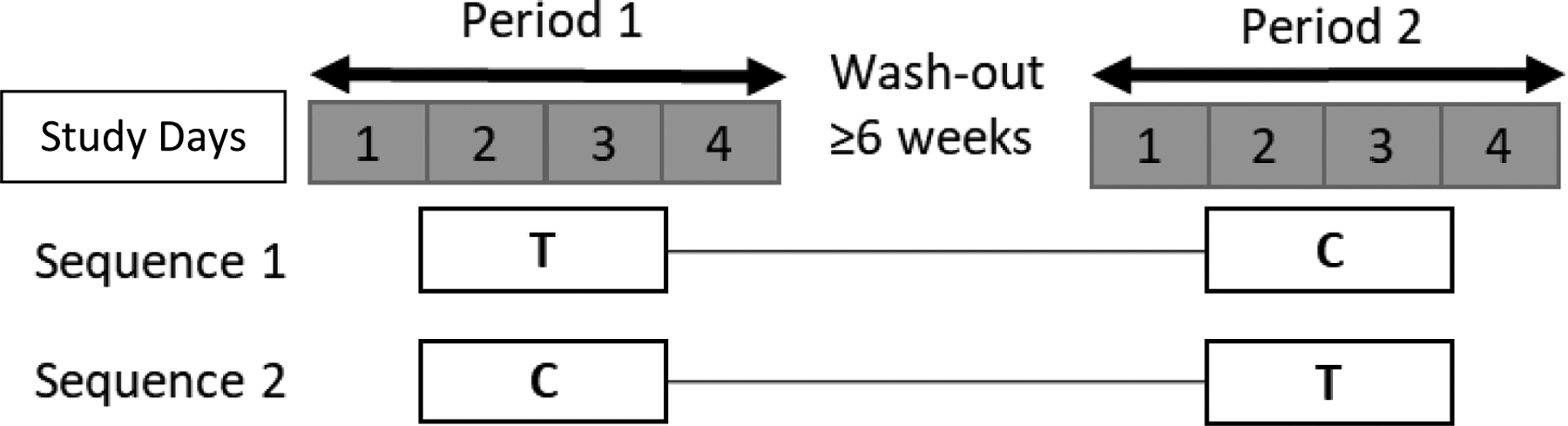
Study Design. Study periods are shown in days 1–4. Study interventions are (1) “T” test +PRO Treatment = 2 g PRO + 600 mg TDF/ 400 mg FTC at t=0 h or (2) “C” control IPERGAY Treatment = 600 mg TDF/ 400 mg FTC at t=0 h; 300 mg TDF/ 200 mg FTC at t= 24, 48 h. Treatment regimens were separated by ≥ 6 weeks wash-out period.
