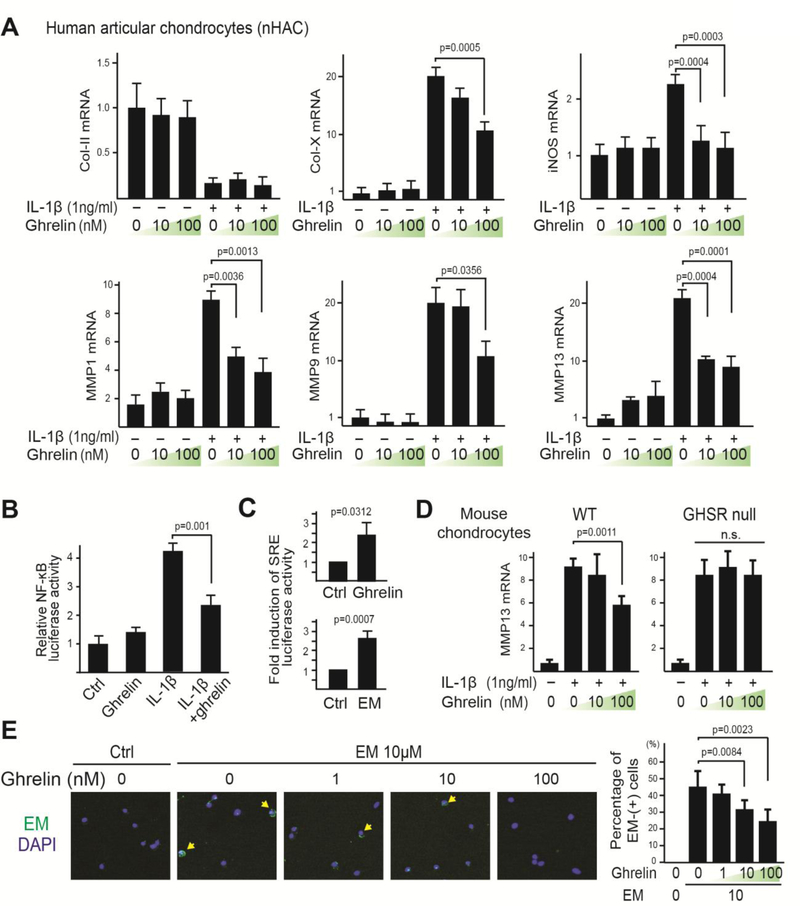
Fig. 3. Ghrelin signaling is necessary and sufficient to inhibit IL-1β-induced catabolic gene expression and NF-κB activation in chondrocytes.
(A) RT-qPCR analysis of Col-II, Col-X, iNOS, MMP1, MMP9 and MMP13 on nHACs treated with ghrelin (0, 10 and 100nM) and IL-1β (1ng/mL). TBP served as a reference gene. (B) NF-κB transactivation assay. nHACs were transiently transfected with NF-κB reporter construct for 24 hours and then treated with ghrelin (10nM) and IL-1β (1ng/mL). A Renilla luciferase construct was co-transfected as an internal control for normalization. (C) Serum Response Element (SRE) luciferase reporter assay. nHACs were transiently transfected with the SRE reporter construct for 24 hours and then treated with ghrelin (10nM) or EM (10μM) for 16 hours. A Renilla luciferase construct was co-transfected as an internal control for normalization. Data are presented as fold activation relative to untreated samples. (D) RT-qPCR analysis of MMP13 on WT and GHSR null mouse chondrocytes treated with ghrelin (0, 10, 100nM) and IL-1β (1ng/mL). TBP served as a reference gene. (E) ICC analysis with an anti-EM antibody on WT chondrocytes co-treated with EM (10μM) and different concentrations of ghrelin (1, 10 and 100nM). Arrows indicate cells with EM staining. Percentage of EM-positive cells was calculated. Data were reported as mean ± SD and analyzed by Dunnett’s test (A, D and E) and an unpaired t-test (B and C). At least three independent experiments were performed. * p<0.05. n.s: not significant.