Figure 3.
TBX-37/38 Are Not Continuously Required for lsy-6 Expression
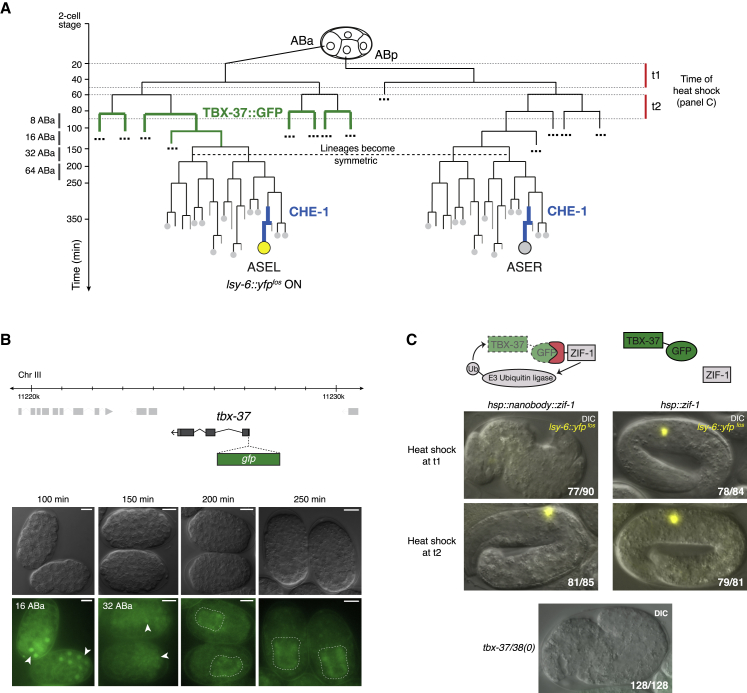
(A) Developmental lineage of ASEL and ASER with relevant timing information for (B) and (C).
(B) Expression of N-terminally tagged, endogenous TBX-37 (or TBX-38, Figure S1) was followed by GFP fluorescence over time. Representative images are shown. Fluorescence was clearly visible at 16 ABa stage (arrowheads) and was dim in a few nuclei at 32 ABa stage but no signal was visible beyond this time point (also Figures S2A and S2B). Autofluorescence from the developing gut is outlined with a dashed line. DIC (differential interference contrast) and fluorescence are shown. Scale bars represent 10 μm.
(C) Degradation of GFP-TBX-37 (in a tbx-38(0) background) was induced using an anti-GFP nanobody fused to the ubiquitin-ligase adaptor ZIF-1, expressed under control of a heat-shock promoter. Representative images show embryos heat shocked at two different times (see A): t1 targeted the peak of TBX-37/38 expression (Figure S2) and caused 77/90 embryos to phenocopy tbx-37/38(0) morphological defects and fail to express lsy-6::yfpfosmid; t2 (onset of degradation 40 min later, Figure S2) caused no defects in morphology or lsy-6 expression in 81/85 embryos. Heat shock of animals expressing ZIF-1 without the targeting nanobody had no effect at either time. Comparison of control and nanobody treatments was done using a chi-squared test: p valuet1 < 0.0001, p valuet2 = 0.297 (Table S1). DIC and fluorescence were overlaid in the same image.