Figure 4. Polar and charged residues close to the middle of the membrane cause trafficking-deficiency.
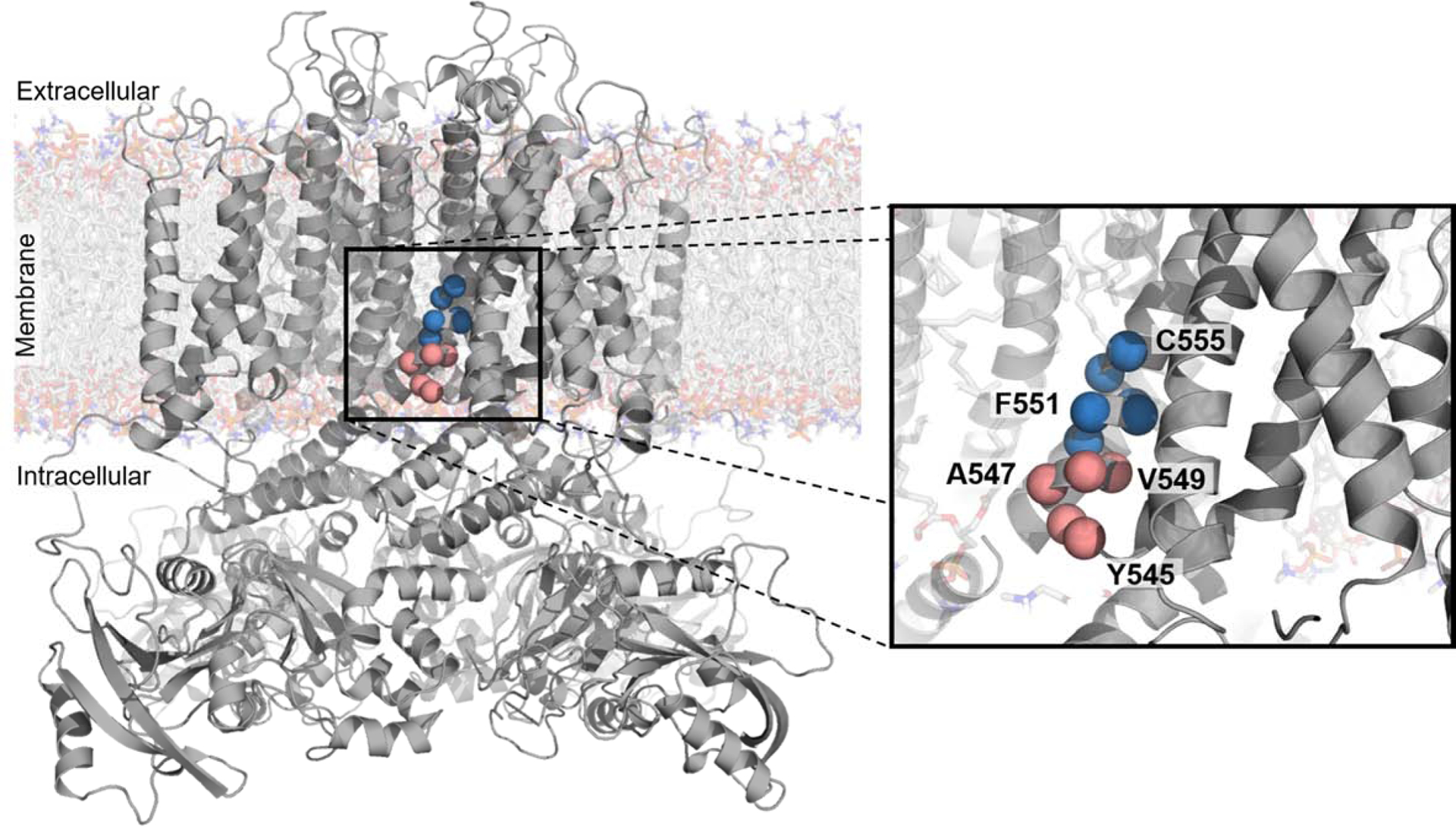
CryoEM structure of KV11.1 (PDB: 5VA1)41 with residues mutagenized in a pilot study of a high-throughput trafficking assay highlighted as red or blue spheres. These residues are on the intracellular half of the S5 transmembrane helix. The red to blue transition from V549 to L550 reflects the transition from residues tolerant of hydrophilic substitution to residues intolerant of hydrophilic substitution, respectively, as observed in the high-throughput trafficking assay (Figure 3B).
