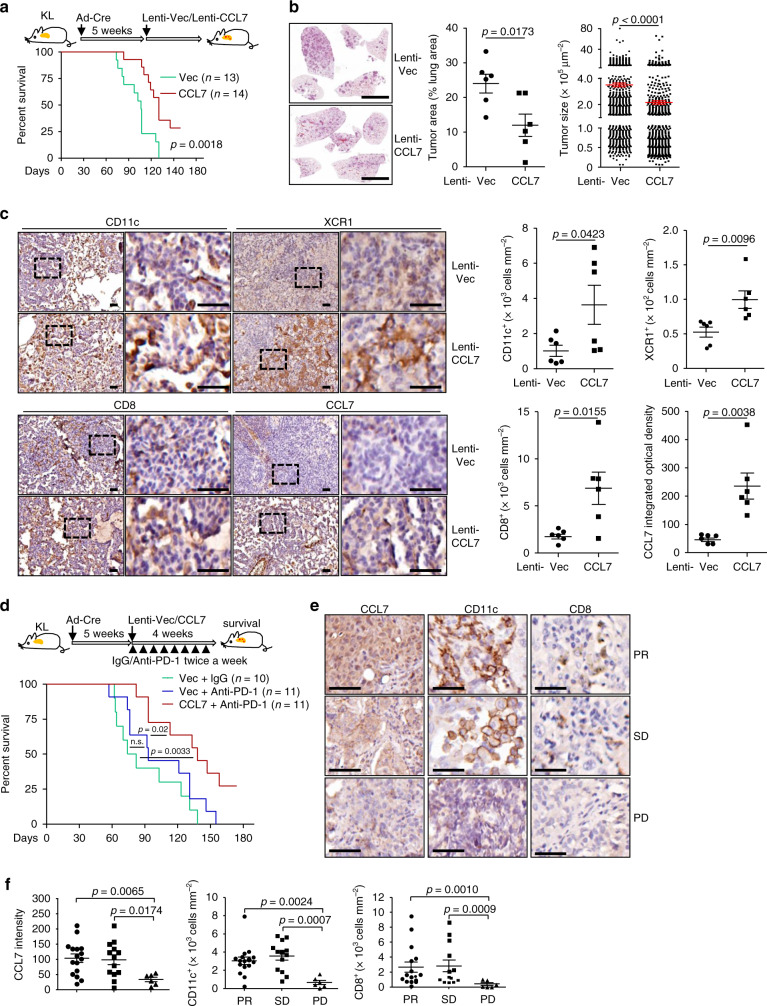
Fig. 8. CCL7 facilitates anti-PD-1 checkpoint immunotherapy in KL mice.
a A scheme (upper) of administration of CCL7 in tumor-burdened KL mice. KL mice were intranasally injected with Ad-Cre (1 × 106 pfu/mouse) for 5 weeks, followed by intranasal injection of Lenti-Vec (n = 13) or Lenti-CCL7 (n = 14) for 5 weeks for analysis or for survival observation. Survival graph (lower) of KL mice treated as described above. b HE staining (left images) and tumor area and size analysis (right graphs) of KL (n = 6 for Vec or CCL7) mice treated as in a. c IHC staining (left images) and intensity analysis (right graphs) of KL (n = 6 for Vec or CCL7) mice treated as in a. d A scheme (upper) and survival analysis (lower graph) of combinational therapy of CCL7 and anti-PD-1 in KL (n = 10, 11, or 11 for Vec + IgG, Vec + anti-PD-1 or CCL7 + anti-PD-1, respectively) mice. KL mice were intranasally injected with Ad-Cre (2 × 106 pfu/mouse) for 5 weeks, followed by intranasal injection of Lenti-Vec or Lenti-CCL7 and intraperitoneal injection of control isotype lgG or anti-PD-1 antibody twice a week for 4 weeks. e, f IHC staining (e) and intensity analysis (f) of CCL7, CD8 and CD11c in CT-guided needle biopsies from patients with advanced NSCLC received anti-PD-1 treatment. PR (n = 16), partial response; PD (n = 6), progressive disease; SD (n = 13), stable disease. Log-rank analysis (a, d), two-tailed student’s t-test (b, c, f). Scale bars, 5 mm (b) or 50 μm (c, e). Graphs show mean ± SEM (b, c, f). Data are combined results of two (b–d) or three (a) independent experiments. Source data are provided as a source data file.