Figure 4. The UPR is activated in HCLE cells subjected to oxidative stress; dynasore inhibits the PERK branch.
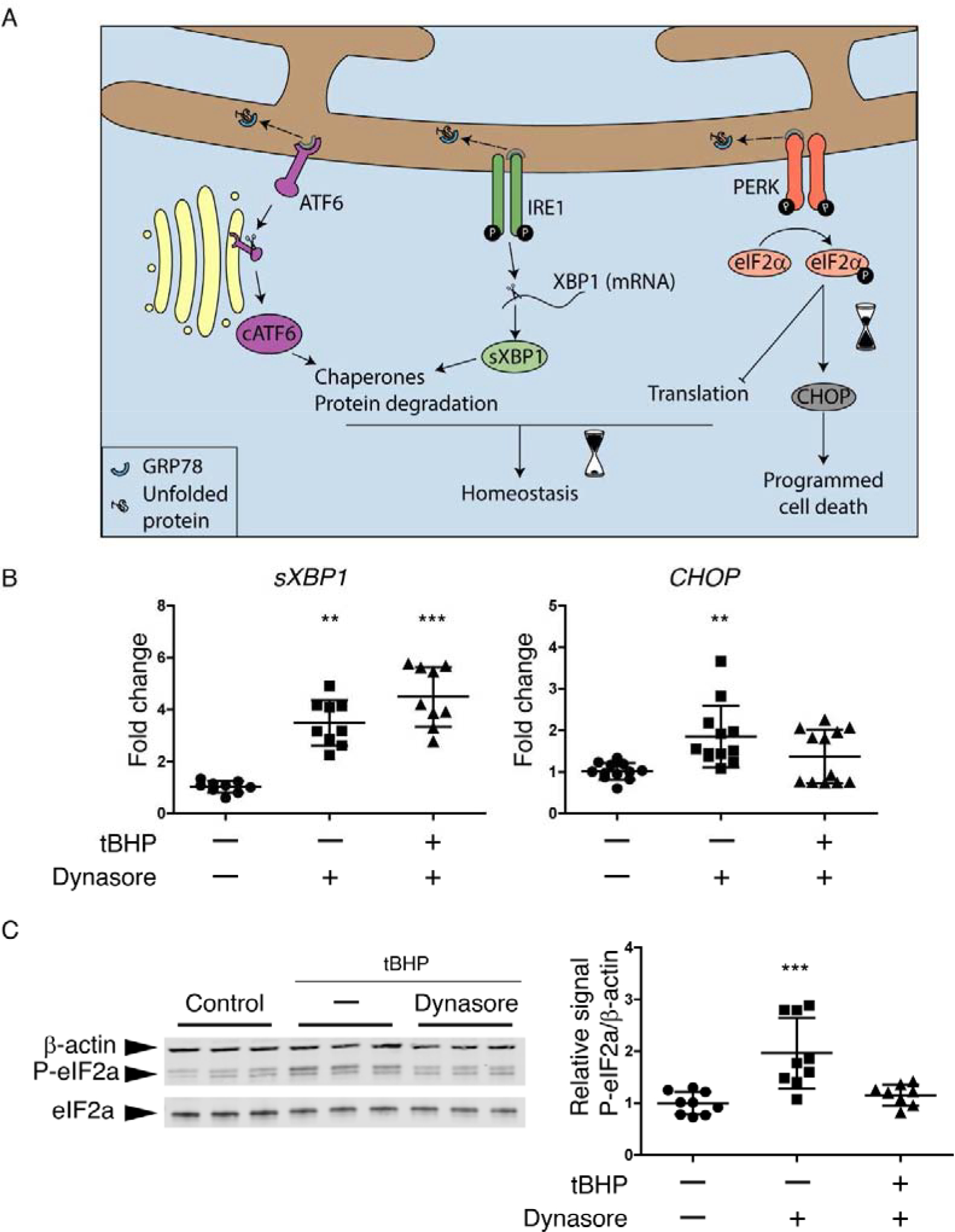
(A) Schematic of the UPR. Dissociation of GRP78 from ATF6, IRE1 and PERK to bind to accumulated unfolded proteins is usually the start point that activates the UPR. The activation of each one of the sensors (ATF6, IRE1 and PERK) produces a second messenger. They main effects of cATF6 and sXBP1 are to increase chaperones production and the activation of ERAD for protein degradation, which can help to recover the homeostasis. While the phosphorylation of eIF2α can reduce protein translation to maintain homeostasis, it can also increase the expression of CHOP when ER stress is prolonged, leading to cell death.
(B and C) Stratified cultures of HCLE cells with mucosal differentiation were exposed to tBHP (10 mM) for 2 hrs while also being treated with 80 μM dynasore or DMSO vehicle. Unstressed cells kept in growth medium with the same DMSO concentration served as control. At the end of the experiment, RNA or protein was isolated.
(B) Relative gene expression of sXBP1 (n = 3) and CHOP (n = 4) was calculated with the 2−ΔΔCt method, using the levels of ACTB expression as housekeeping and the expression in control cells as the calibrator.
(C) Representative images of western blot for P-eIF2α, eIF2α and ACTB and densitometry analysis of P-eIF2α normalized to β-actin levels as a loading control. n = 3.
The data are presented as mean ± standard deviation. Significant differences were determined using the Kruskal-Wallis test with Dunn’s post-hoc test. ** P<0.01; *** P<0.001.
