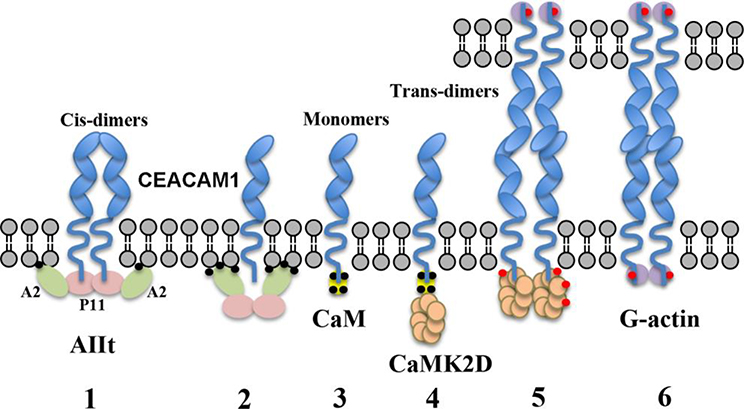
Figure 8. Scheme showing proposed steps controlling the conversion of cis- to trans-dimers in CEACAM1-SF.
1: The cis-dimer state is stabilized by the interaction of two short cytoplasmic domains with AIIt. According to other studies, only Annexin 2 (A2) interacts with the membrane in a calcium dependent manner. 2: When intracellular calcium (black dots) levels rise, AIIt undergoes a conformation change that pushes S100A10 away from the membrane, disrupting its interaction with CEACAM1-SF. 3: The cytoplasmic domain of CEACAM1 now binds to Ca2+/CaM (yellow). 4–5: CaMK2D (beige) is recruited to the Ca2+/CaM-CEACAM1-SF complex where it begins its autoactivation cycle and phosphorylates (red dots) CEACAM1-SF on Thr-457. 6: CaMK2D dissociates from CEACAM1-SF and allows G-actin (purple) binding. Not shown: G-actin polymerizes, stabilizing the formation of trans-dimers. (Ca2+ indicated by small black circles, phosphorylation by small red circles).