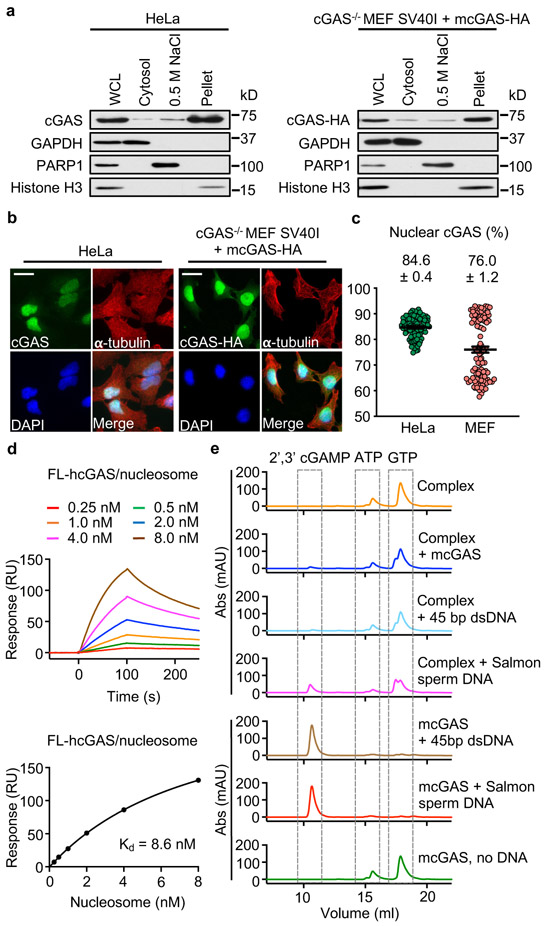
Figure 1. Tight nucleosome binding inactivates cGAS.
a. Western blot analyses of cGAS expression in 1% SDS whole cell lysates (WCL), 0.2% NP-40 cytosolic fractions, 0.5 M NaCl nuclear extracts, and 1% SDS nuclear lysates of HeLa and cGAS−/− MEF SV40I + mcGAS-HA cells.
b. Immunofluorescence microscopy images of HeLa and cGAS−/− MEF SV40I + mcGAS-HA cells stained with anti-cGAS or –HA, and -α-tubulin antibodies and DAPI. The scale bars denote 50 μm.
c. The percentage of nuclear- to whole cell-cGAS ratio quantified by microscopy. All data are presented as mean ± SEM.
d. Surface plasmon resonance (SPR) shows that full-length human cGAS binds to in vitro reconstituted human nucleosome with nanomolar affinity. The binding affinity (Kd) was determined by fitting the binding data to a simple one-to-one binding model.
e. Enzyme activity assays by ion exchange chromatography show that nucleosome binding potently inhibits the catalytic activity of cGAS.