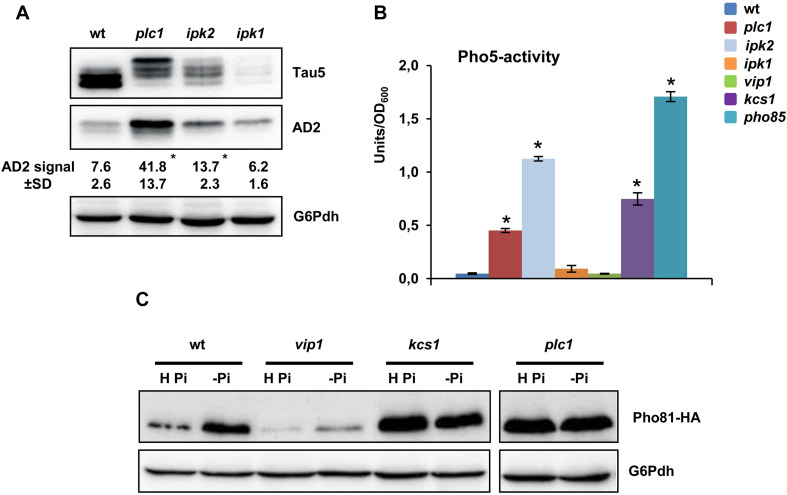
FIGURE 4.
Tau phosphorylation is regulated by IP signaling by Pho85-dependent and Pho85-independent mechanisms. (A) Protein extracts from SCD-grown cultures of wild-type (wt), plc1, ipk2, and ipk1 transformants of the CEN.PK2-1C yeast background, which express a native isoform of tau, were analyzed by Western blot using Tau5 and AD2 antibodies. The numbers show the AD2 band intensity (±SD) expressed as arbitrary units. Quantification was carried out as in Figure 1C. Data are the mean (±SD) of three independent experiments. *p < 0.05 for AD2 band intensitiy of the mutant strains compared with AD2 band intensity of the wild-type control strain. (B) The activity of the Pho85-repressible acid phosphatase Pho5 (units/OD600) was measured in protein extracts from logarithmic-SCD-Ura-grown (Abs600 ∼0.3–0.5) cultures. Cells of the wild-type (wt), plc1, ipk2, ipk1, vip1, kcs1, and pho85 mutants were analyzed. Data represent the mean value (±SD) of three independent experiments. *p < 0.05 for Pho5 activity of the mutant strains compared with the Pho5 activity of the wild-type control strain. (C) The abundance of Pho81 in Pho81-HA-tagged cells of the CEN.PK2-1C wild-type, vip1, kcs1, and plc1 mutant strains was measured before and after transfer for 3 h of yeast cells from high-phosphate (H Pi) to phosphate-free medium (-Pi). Antibodies against HA and glucose-6-phosphate dehydrogenase (G6Pdh; loading control) were used. A representative experiment is shown.