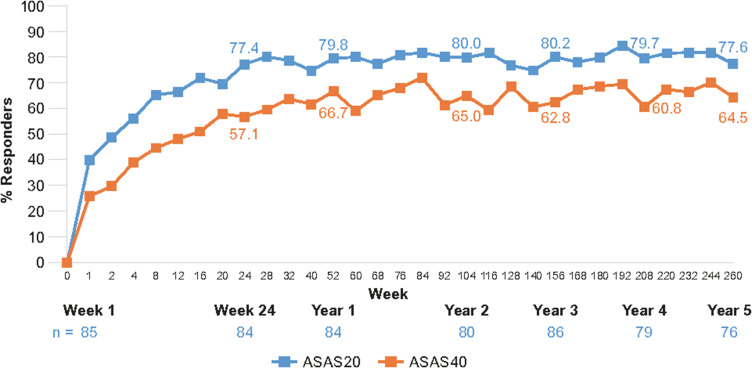
Figure 3.
ASAS20 and ASAS40 line graphs showing response rates with secukinumab 150 mg through 5 years in MEASURE 1.37
Notes: Data shown are as observed through 5 years for patients who entered the three-year extension study after the initial two-year study, and do not include placebo switchers or patients whose dose was escalated. Reproduced from Baraliakos X et al. Long-term efficacy andsafety of secukinumab 150 mg in ankylosing spondylitis: 5-yearresults from the phase III MEASURE 1 extension study. RMD Open. 2019;5(2):e001005, © 2019. With permission from BMJ Publishing Group Ltd.
Abbreviations: ASAS, Assessment of SpondyloArthritis international Society response criteria.