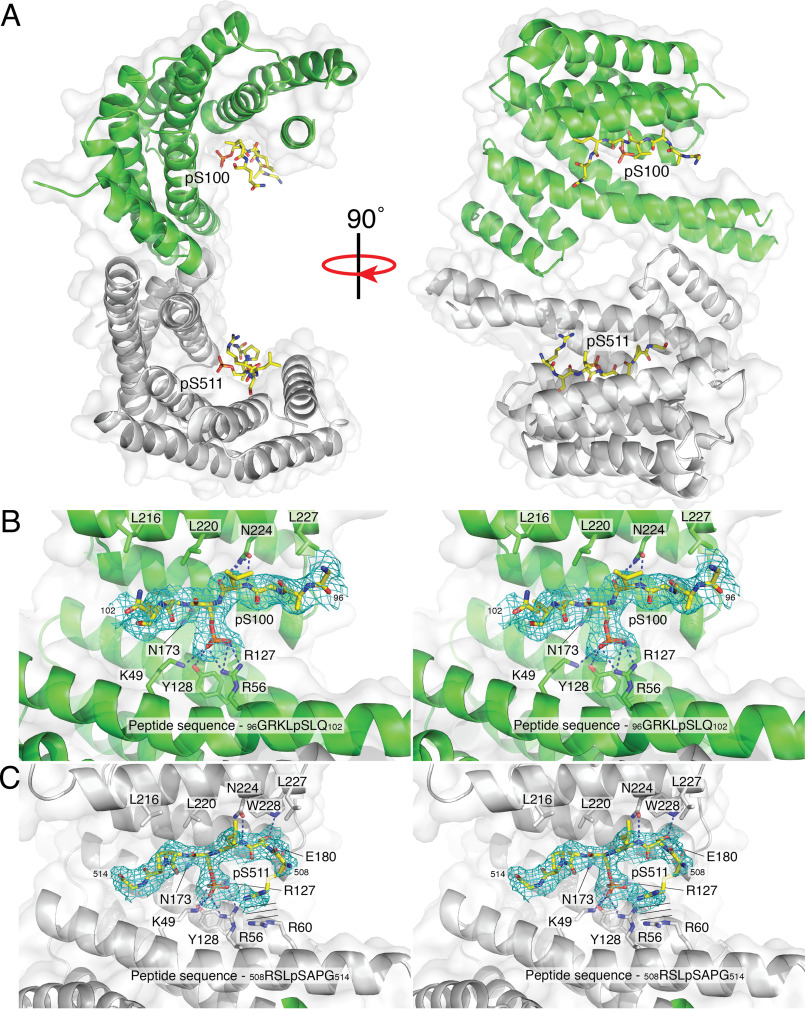
Figure 5.
Structure of a 14-3-3 diphosphopeptide complex. A, surface representation of the 14-3-3 dimer (monomers containing the pSer100 and pSer511 binding sites are shown in green and gray, respectively) with secondary structure shown in ribbons. The pSer100 and pSer511 ends of the diphosphorylated peptide are shown as sticks in yellow. B, stereo image of difference maps of the pSer100 binding site. C, stereo image of difference maps of the pSer511 binding site. The parallel lines represent the parallel stacking interactions between Arg508 on the diphosphopeptide and Arg60 on 14-3-3.