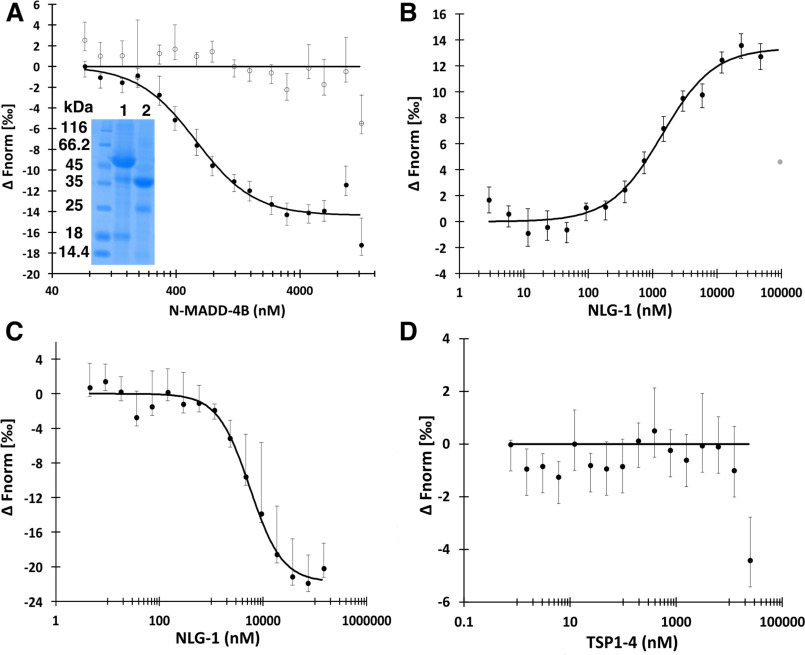
Figure 4.
N-MADD-4B interacts with NLG-1 through the Ig-like domain. A, N-MADD-4B (73 nm to 12.5 μm) comprising mostly the intact protein (see lane 1 of inserted SDS-polyacrylamide gel (same gel as in Fig. 3A)) binds to labeled NLG-1 (35 nm) with an estimated Kd of 578 ± 77 nm (circles, n = 4) whereas N-MADD-4BΔIg (lane 2 of inserted SDS-polyacrylamide gel) does not bind (empty circles, n = 3). B, NLG-1 (38 nm to 125 μm) competes with labeled NLG-1 (35 nm) for binding to N-MADD-4B (1.25 μm) with an estimated Kd of 1396 ± 285 nm (n = 3). One outlier data point excluded from the fit is displayed in gray. C, NLG-1 (4.5 nm to 140 μm) interacts with the labeled Ig-like domain (150 nm) with an estimated Kd of 5580 ± 825 nm (n = 3). D, TSP1–4 (3.5 nm to 25 μm) does not interact with labeled NLG-1 (35 nm) (n = 3). Error bars, S.D. All fit parameters are summarized in Table 2.