Figure 5.
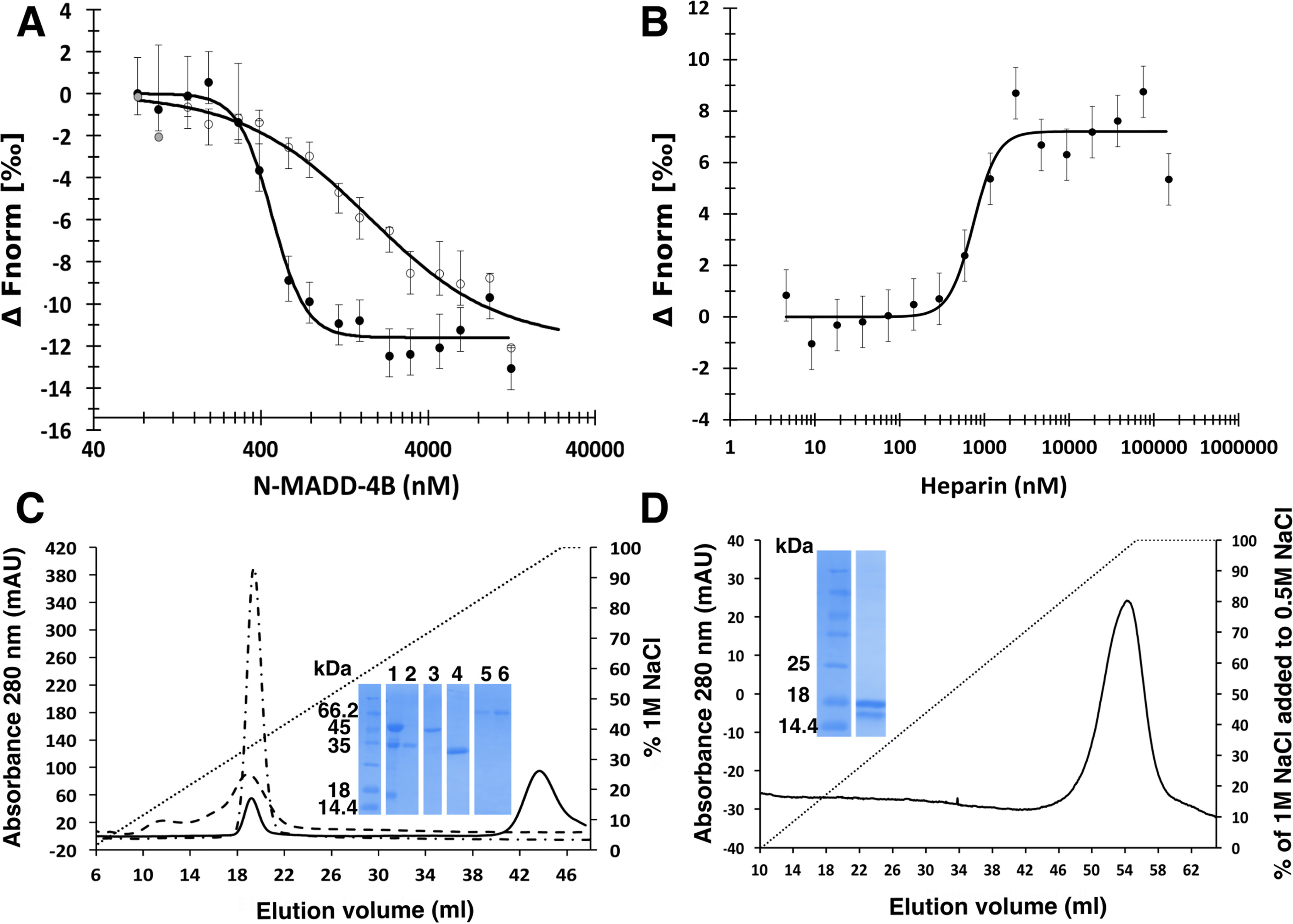
Heparin challenges the N-MADD-4B interaction with NLG-1. A, binding of N-MADD-4B (73 nm to 12.5 μm) to labeled NLG-1 (35 nm) (filled circles) is compromised in the presence of heparin (250 μm) (empty circles). Three outlier data points excluded from the fit are displayed in gray. B, heparin (4.5 nm to 150 μm) prevents binding of N-MADD-4B (1 μm) to labeled NLG-1 (35 nM) with a half-effect at ∼740 nm. C, relative affinities of N-MADD-4B fragments (solid line), TSP1–4 (dash-dotted line), and NLG-1 (dashed line) for immobilized heparin using NaCl elution (0–1 m gradient over 8 CV) and conductivity recording. Inset, SDS-PAGE of N-MADD-4B input (lane 1) and of the peak elution fractions of N-MADD-4B fragment (lane 2), intact MADD-4B (lane 3), TSP1–4 (lane 4), and NLG-1 (lanes 5 and 6). Intact N-MADD-4B is retained much more strongly (elution at 82 mS/cm; i.e. 900 mm NaCl) than N-MADD-4BΔIg (343 mm NaCl; i.e. 22.6 mS/cm), TSP1–4 (349 mm NaCl; i.e. 21.6 mS/cm), or NLG-1 (337 mm NaCl; i.e. 21.9 mS/cm). D, relative affinity of the isolated Ig-like domain for immobilized heparin (0.5–1.0 m NaCl gradient over 9 CV). The Ig-like domain is as strongly retained as intact N-MADD-4B. Inset, SDS-PAGE of the Ig-like elution fraction in which partial degradation is visible. (All proteins were loaded on the heparin column as 0.5 ml at 0.8 mg/ml.)
