Figure 8.
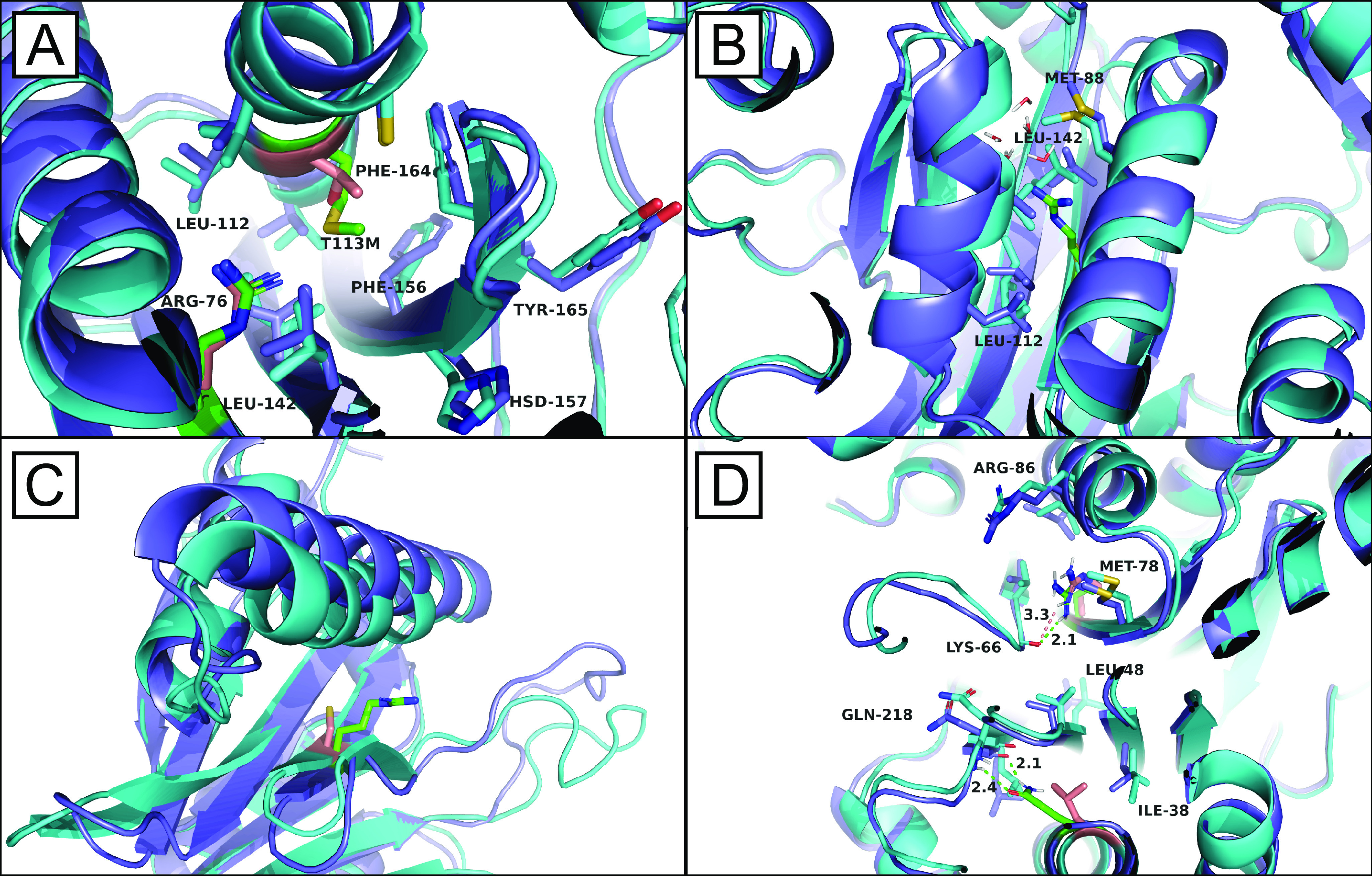
Structural observations from the MSλD trajectories. A, overlay of complex C76R Pup2 (cyan with residues 76 and 113 in peach) and complex C76R+T113M pup2-ts (purple with residues 76 and 113 in green). Arg-76 points inward, disrupting important hydrophobic interactions between Leu-144, Leu-112, Ile-144, Ile-70, and Val-116. Met-113, however, is able to occupy space adjacent to Arg-76 to help offset the disruption of this hydrophobic core in Pup2. B, overlay of complex native Pup2 (cyan) and complex C76R pup2-ts (purple with residue 76 in green). Nearby water molecules are able to penetrate into the core of Pup2 when Arg-76 protrudes inward and disrupts the hydrophobic network of nearby valine, leucine, and isoleucine residues. C, overlay of unbound native Pup2 (cyan with residue 76 in peach) and unbound C76R pup2-ts (purple with residue 76 in green). In addition to its inward conformation, Arg-76 is also observed to protrude outward, which induces large structural distortions in residues 84–101, the α-helix positioned above Arg-76, and 53–71, consisting of part of a β-sheet and a flexible loop. D, overlay of complex C76R Pup2 (cyan with residues 76 and 204 shown in peach) and complex C76R+L204Q pup2-ts (purple with residues 76 and 204 shown in green). As an interesting example of stabilizing complementarity, when Arg-76 points outward, Gln-204 (green) can hydrogen-bond to the backbone of Gln-218 to help stabilize nonnative fluctuations in the bottom half of Pup2.
