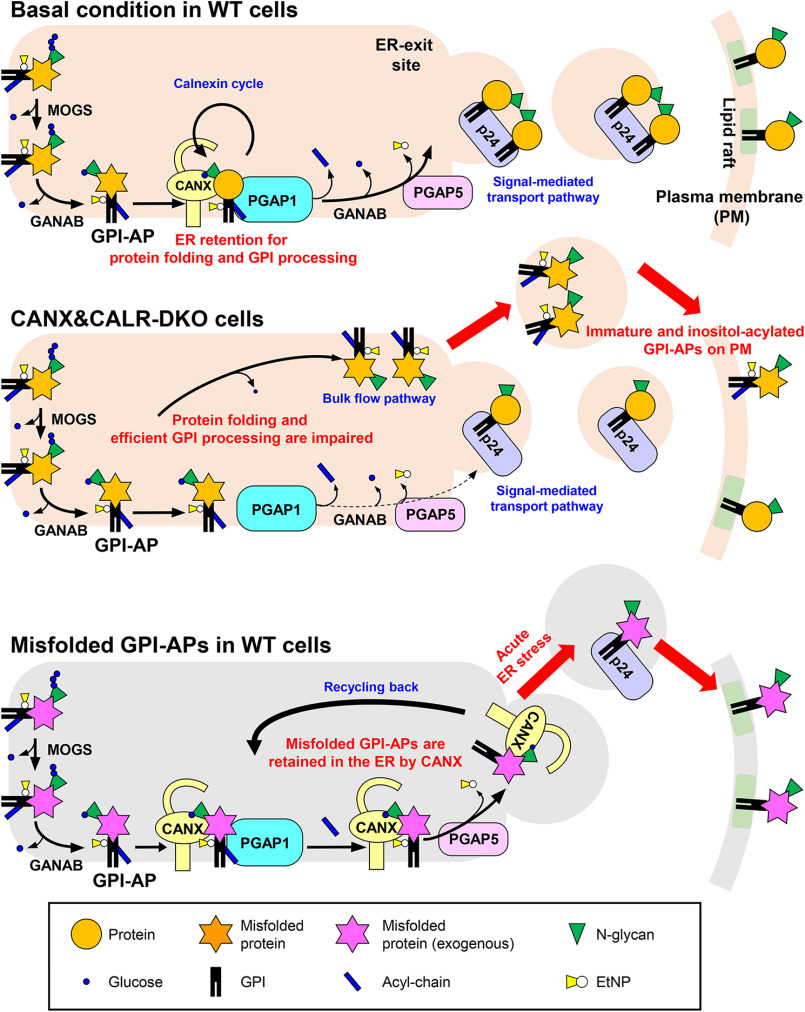
Figure 9.
Summary models for folding and inositol deacylation of GPI-APs regulated by calnexin/calreticulin. Top, under normal conditions, N-glycans and GPI are transferred to the newly synthesized GPI-APs. After glucose trimming by α-glucosidase I and II, the N-glycan structure becomes Glc1Man9GlcNAc2, which is specifically recognized by calnexin/calreticulin. By directly binding with calnexin, immature GPI-APs enter the ER quality control system and finally become mature GPI-APs. In addition, calnexin retains GPI-APs in the ER and associates with PGAP1, which is required for the efficient GPI-inositol deacylation. After the GPI moiety is remodeled by PGAP1 and PGAP5, GPI-APs are transported from the ER to the Golgi by the cargo receptors, the p24 family of proteins. Middle, in CANX&CALR-DKO cells, there is no calnexin/calreticulin-dependent ER quality control system and ER retention system. GPI-APs exit the ER with incomplete protein folding and GPI remodeling. Bottom, in WT cells, under basal conditions, misfolded GPI-APs are retained in the ER by calnexin. During the long time being retained in the ER, an acyl-chain on the GPI-inositol is removed by PGAP1. Once acute ER stress is induced, deacylated and misfolded GPI-APs are quickly transported by p24 family members and exposed on the plasma membrane.