Figure EV2. Interaction of VAP‐A, VAP‐B, and MOSPD2 with the Phospho‐FFAT of STARD3.
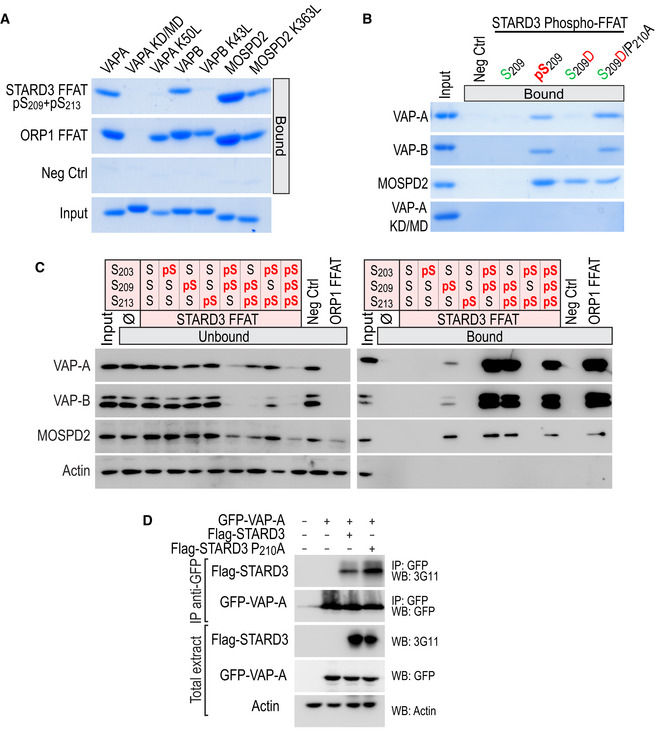
- Interaction of VAP‐A K50L, VAP‐B K43L, and MOSPD2 K363L mutants with conventional and Phospho‐FFAT motif. Wild‐type and mutant MSP domains were pulled down with phosphorylated STARD3 (pS209 + pS213), ORP1 FFAT peptides, and with the negative control peptide. The input fraction corresponds to the recombinant proteins used in the assay. Input and bound proteins were revealed by Coomassie Blue staining. Note that lysine K50 in VAP‐A and K43 in VAP‐B are required for the interaction with a Phospho‐FFAT, and not with a conventional FFAT.
- Interaction of VAP‐A, VAP‐B, MOSPD2 with phosphomimetic STARD3 Phospho‐FFAT motif. Wild‐type and mutant MSP domains were pulled down with unphosphorylated, phosphorylated (pS209), phosphomimetic (S209D and S209D/P210A), and with the negative control peptide. The input fraction corresponds to the recombinant proteins used in the assay. Input and bound proteins were revealed by Coomassie Blue staining. Acidic (D) or phosphorylated residues (pS), and alcoholic (S) residues are in red and green, respectively; the other residues are in black.
- STARD3 and VAP‐A/VAP‐B or MOSPD2 complex formation requires a unique phosphorylation of the Phospho‐FFAT motif. Western blot analysis of proteins pulled down using the peptides described in Fig EV3A. The input fraction corresponds to the HeLa cell total protein extract. The streptavidin beads were first coupled to the indicated biotinylated peptides, or left without peptide (ø). The soluble fraction after the incubation of the protein extract with the beads (Unbound; left), and proteins attached to the beads (Bound; right) were analyzed by Western blot using anti‐VAP‐A, anti‐VAP‐B, and anti‐MOSPD2 antibodies. Actin was used as a loading control.
- Immunoprecipitation (GFP‐Trap) experiments between GFP‐tagged VAP‐A and Flag‐tagged STARD3 (WT and P210A mutant). Approximatively 15 µg of total protein extracts was analyzed by Western blot using anti‐STARD3, anti‐GFP, and anti‐Actin antibodies. Immunoprecipitated material was analyzed using anti‐STARD3 and anti‐GFP antibodies.
Source data are available online for this figure.
