Table 2.
HIV-Related Outcomes (%) for Patients With an Elevated Viral Load, Comparing Those Above and Below Threshold of 1,000 copies/mL (n = 191,764), South Africa, 2013–2015
| Viral Load Test Result |
Comparison of Viral Load Test Result of 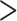 1,000 vs. 1,000 vs. 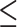 1,000 copies/mL 1,000 copies/mL
|
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Just Below 1,000 copies/mL | Just Above 1,000 copies/mL | ITT RD a | ITT 95% CI | Bandwidth, copies/mL | CACE RD b (RD/First-Stage RD) | CACE 95% CI |
| Repeat viral load testing within 6 months (first-stage RD) | 24.8 | 32.8 | 8.0 | 6.2, 9.7 | 197.6 | ||
| Retained at 12 months | 62.1 | 65.0 | 2.9 | 0.6, 5.2 | 136.0 | 38.1 | 7.7, 68.4 |
| Retained at 18 monthsc | 58.5 | 60.0 | 1.6 | −0.8, 4.0 | 161.9 | 19.9 | −10.0, 49.8 |
| Retained at 24 monthsd | 54.7 | 54.6 | −0.1 | −2.8, 2.5 | 183.4 | 1.5 | −28.4, 31.4 |
| Retained and suppressed at 12 monthse | 35.8 | 41.6 | 5.8 | 3.0, 8.6 | 93.7 | 85.2 | 35.9, 100.0 |
| Retained and suppressed at 18 monthsc,e | 36.3 | 38.5 | 2.2 | −0.2, 4.6 | 150.6 | 31.1 | −2.6, 64.8 |
| Retained and suppressed at 24 monthsd,e | 36.6 | 36.7 | 0.1 | −2.3, 2.5 | 202.2 | 1.5 | −28.3, 31.4 |
| Transferred clinics at least once | 18.6 | 18.9 | 0.3 | −1.4, 2.1 | 152.4 | 4.3 | −19.2, 27.8 |
Abbreviations: CACE, complier average causal effect; CI, confidence interval; ITT, intention-to-treat; RD, risk difference.
a Difference between viral load tests just above 1,000 cells/mL and viral load tests just below 1,000 cells/mL. Data-driven Calonico, Cattaneo, and Titiunik (21) bandwidth obtained using rdrobust package in STATA.
b CACE estimates use outcome optimal bandwidth (not first-stage bandwidth). CACE RDs and 95% CIs estimated using 2-stage least squares regression. The CACE is estimated as a linear probability model, which has no constraints on generating CIs outside the bounds of 0 and 1.0. Square brackets are used where the CACE 95% CI exceeds the logical bounds of 1.0. First-stage RD represents the RD in repeat testing within 6 months for viral loads just above and just below 1,000 copies/mL.
c 151,703 viral load values had at least 24 months of follow-up time and were included in 18-month outcomes.
d 113,617 viral load values had at least 30 months of follow-up time and were included in 24-month outcomes.
e Negative outcome is having a viral load test in that specified time interval that is ≥400 copies/mL. Excludes patients with no viral load test in specified time interval.
