FIGURE 1.
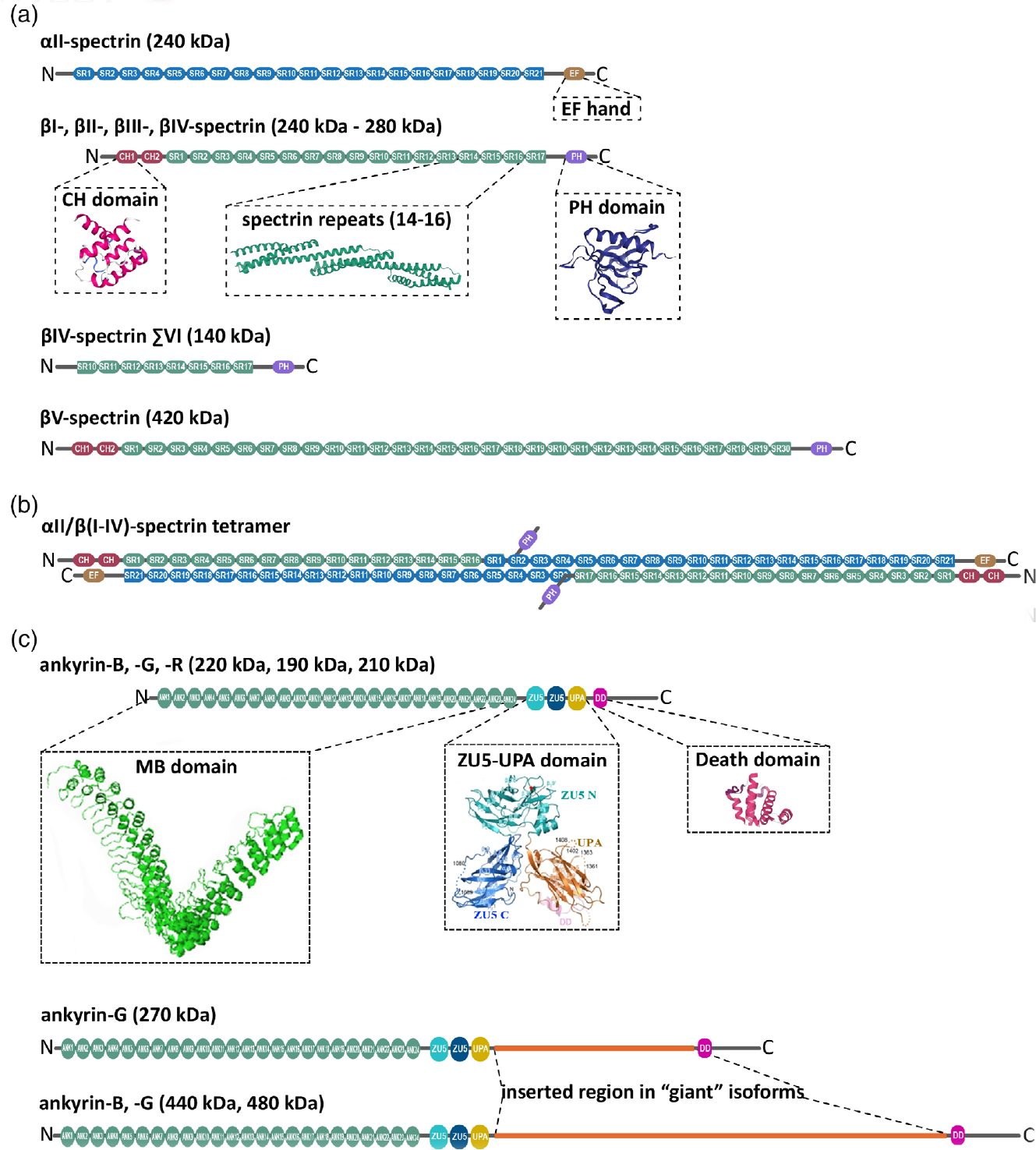
Structure domains of neuronal spectrins and ankyrins. (a) αII-spectrin spans 21 modular spectrin repeats (SR, blue), followed by a calcium-binding EF hand domain (light brown) close to the C-terminus. αII-spectrin also contains a Src-homology 3 (SH3) domain in SR9, and a calmodulin-binding loop in SR11. Neuronal spectrins also include canonical βI-, βII-, βIII-, and βIV-spectrins, which are comprised of 17 SR (green; ribbon diagram of the crystal structure of SR14–16 from Grum, MacDonald, & Mondragón, 1999), two in tandem calponin homology domains (CH, red; ribbon diagram of the crystal structure from Djinović-Carugo, Bañuelos, & Saraste, 1997) close to the N-terminus, and a pleckstrin homology domain (PH, purple; ribbon diagram of the crystal structure from Lemmon, Ferguson, & Abrams, 2002). βV-spectrin, the largest known mammalian spectrin, contains 13 additional SR. Alternatively spliced βIV-spectrin ΣVI, which lacks the PH domain and the first 9 SR, is a well-studied noncanonical, neuronal β-spectrin isoform. (b) αII-spectrin uses its N-terminus SR to bind the N-terminus SR of β-spectrins and associate as heterodimers. These heterodimers use antiparallel head-to-head association to form tetrameric units assembles with actin and networks with long-range regularity. (c) Canonical neuronal ankyrins contain a membrane-binding domain comprised of 24 ankyrin repeats (ANK) in the N-terminus (MBD, green; ribbon representation of the crystal structure from Michaely, Tomchick, Machius, & Anderson, 2002), followed by a supermodule that contains two ZU5 domains a UPA domain (teal, blue, orange; ribbon representation of crystal structure from Wang, Yu, Ye, Wei, & Zhang, 2012), which contains the ankyrin-binding site, a death domain (pink; ribbon representation of crystal structure from Wang, Simon, et al., 2019; Wang, Taki, et al., 2019) and a C-terminal unstructured regulatory domain (gray). The giant, neuron-specific ankyrin isoforms have an insertion of a single exon (orange) after the UPA domain
