FIGURE 2.
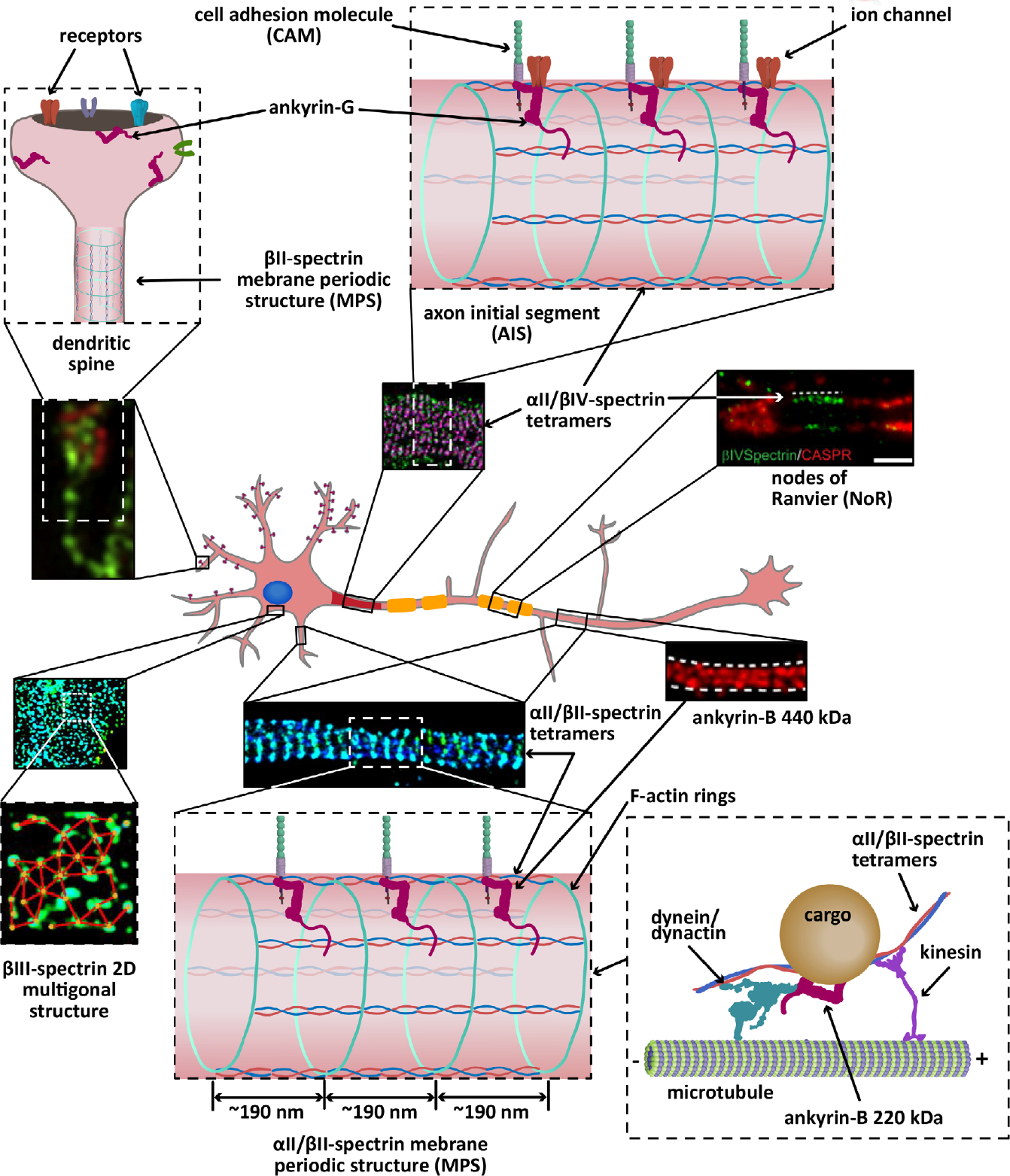
Cartoon representation of the localization and organization of ankyrins and spectrins in a hippocampal neuron. αII/βII-spectrin tetramers crosslink actin rings to line the axon (STORM image adapted from Zhong et al., 2014), parts of the shafts of dendrites, and a portion of spine necks (not shown), all with an average spacing regularity of ~190 nm. Both αII-spectrin and βII-spectrin also associate with microtubule motors to promote axonal transport of synaptic vesicles. The 440 kDa ankyrin-G isoform, which also shows long range periodicity (STED image adapted from Yang, Walder-Christensen, Kim, et al., 2019; Yang, Walder-Christensen, Lalani, et al., 2019), likely binds the periodic axonal βII-spectrin to stabilize cell adhesion molecules, such as L1CAM. Ankyrin-B 220 kDa (not shown) couples the retrograde transport machinery to organelles to facilitate axonal transport. Like βII-spectrin, βIII-spectrin forms periodic quasi-1D structures in dendritic processes (not shown), in addition to 2D polygonal lattices in the soma (STORM image adapted from Han et al., 2017, with permission). Similarly, αII/βIV-spectrin tetramers form periodic quasi-1D structures in the axon initial segment (AIS) (STORM image adapted from Huang, Zhang, Ho, et al., 2017) and nodes of Ranviers (NoR) (STED image adapted from D’Este, Kamin, Balzarotti, & Hell, 2017, with permission) in myelinated axons, where it binds ankyrin-G to stabilize cell adhesion molecules and ion channels. Ankyrin-R and βI-spectrin concentrate at the nodes to stabilize ion channels (not shown). βI-spectrin is also found in the soma, dendrites, and spines (not shown). Ankryin-G also localizes to dendritic spines (SIM image adapted from Smith et al., 2014, with permission), where it is required for the retention of AMPA receptors
