Figure 4.
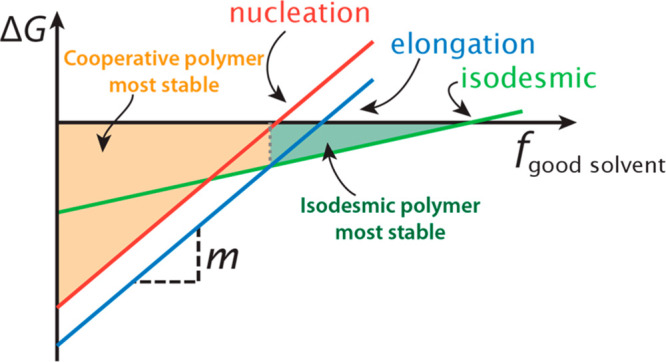
Schematic representation of the changes in Gibbs free energies (ΔG) upon the addition of a good solvent of the various aggregation pathways in a competitive supramolecular polymerization involving a cooperative and isodesmic pathway. As a fraction of good solvent, fgood-solvent, is added, the change in stability of the aggregates is given by their m-value. When the elongation or isodesmic pathway is lower in ΔG, the cooperative or isodesmic polymers, respectively, are the most stable polymers, as indicated by the shaded areas and dashed lines.
