Fig. 3.
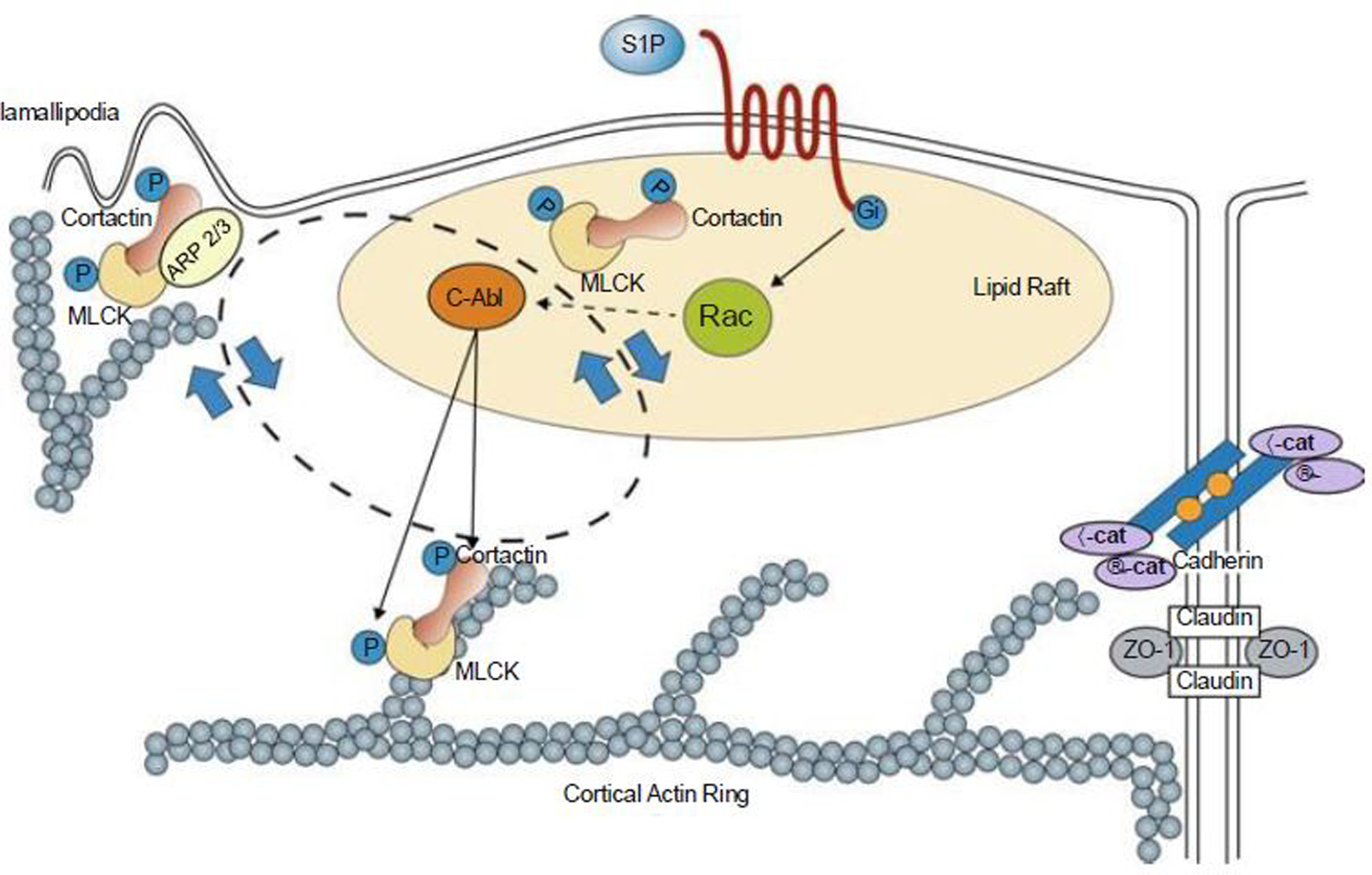
S1P regulates enhanced EC barrier function. Ligation of the S1PR1 Gi protein-coupled receptor by S1P rapidly (within 1–5 min) activates Rac and recruits signaling molecules and cytoskeletal effectors such as c-Abl, cortactin, and nmMLCK to lipid rafts (or CEMs). Tyrosine phosphorylation of these molecules is observed both in lipid rafts and at the EC periphery in association with cortical actin and lamellipodia formation. This activated complex likely interacts with Arp 2/3 machinery to produce lamellipodia protrusion at the cell periphery, which serves to increase overlap between adjacent ECs. The initiation and precise sequence of events responsible for these protein movements are unclear, but within 5 min after S1P stimulation, these proteins are found simultaneously distributed in lipid rafts, cortical actin structures, and peripheral membrane ruffling/lamellipodia (indicated by the bidirectional circle). S1P also induces adherens junction (AJ) and tight junction (TJ) assembly that serve to further strengthen the endothelial barrier. Multiple other signaling and cytoskeletal effector molecules participate in this process as reviewed elsewhere (Wang and Dudek 2009). MLCK non-muscle myosin light-chain kinase, VE-cad vascular endothelial cadherin, ZO-1 zona occluden protein-1 [Modified from Belvitch and Dudek (2012)]
