Abstract
Increased activity of signal transducer and activator of transcription 3 (STAT3) is common in human malignancies, including colorectal cancers (CRCs). We have recently reported that STAT3 gene expression correlates with resistance of CRC cell lines to 5-fluorouracil (5-FU)-based chemoradiotherapy (CT/RT). This is of considerable clinical importance, because a large proportion of rectal cancers are resistant to preoperative multimodal treatment. To test whether STAT3 contributes to CT/RT-resistance, we first confirmed that STAT3 protein expression correlated positively with increasing resistance. While STAT3 was not constitutively active, stimulation with interleukin-6 (IL-6) resulted in remarkably higher expression levels of phosphorylated STAT3 in CT/RT-resistant cell lines. A similar result was observed when we determined IL-6-induced expression levels of phosphorylated STAT3 following irradiation. Next, STAT3 was inhibited in SW480 and SW837 using siRNA, shRNA and the small-molecule inhibitor STATTIC. Successful silencing and inhibition of phosphorylation was confirmed using Western blot analysis and a luciferase reporter assay. RNAi-mediated silencing as well as STATTIC treatment resulted in significantly decreased clonogenic survival following exposure to 3 μM of 5-FU and irradiation in a dose-dependent manner, with dose-modifying factors of 1.3–2.5 at a surviving fraction of 0.37. Finally, STAT3 inhibition led to a profound CT/RT-sensitization in a subcutaneous xenograft model, with a significantly delayed tumor regrowth in STATTIC-treated mice compared with control animals. These results highlight a potential role of STAT3 in mediating treatment resistance and provide first proof of concept that STAT3 represents a promising novel molecular target for sensitizing resistant rectal cancers to CT/RT.
Keywords: rectal cancer, chemoradiotherapy-resistance, chemoradiotherapy-sensitization, STAT3, molecular target
Aberrant activation of members of the signal transducer and activator of transcription (STAT) family is a recurrent finding in many human malignancies.1,2 In general, STATs carry extracellular signals from various cytokine and growth factor receptors to the nucleus and, accordingly, numerous oncogenic signaling pathways converge at this point. The most studied member of this protein family is STAT3, which functions as an oncogene.3 Upon recruitment and tyrosine phosphorylation (pSTAT3Tyr705), monomeric STAT3 dimerizes and translocates to the nucleus, where it tightly regulates several fundamental transcriptional programs.4 The tumor-promoting features of STAT3 signaling are mediated through increased tumor cell proliferation and neo-angiogenesis, through suppression of antitumor responses of the tumor-associated stroma, and through stimulation of tumor-promoting inflammation.5–7 Consequently, STAT inhibition holds great promise for future treatment concepts in oncology.8
Recently, we reported that STAT3 gene expression, determined by microarray analysis, was positively correlated with resistance of colorectal cancer (CRC) cell lines to 3 μM of 5-fluorouracil (5-FU) and 2 Gy of irradiation, assessed in colony-forming assays.9 This is of high clinical relevance, because a substantial percentage of primary rectal cancers is resistant to preoperative multimodal treatment, which represents the standard therapy in locally advanced stages of this disease.10,11 We therefore tested whether this increased expression of STAT3 has a functional role in mediating resistance to chemoradiotherapy (CT/RT).
Material and Methods
Cell lines and cell culture
Human CRC cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in their recommended media, supplemented with 10% fetal bovine serum (Pan, Aidenbach, Germany) and 2 mM L-glutamine (BioWhittaker, Verviers, Belgium). Periodically, mycoplasma contamination was excluded using the MycoAlert® Mycoplasma Detection Kit (Lonza, Cologne, Germany), and cell-line cross-contamination was excluded using short tandem repeat profiling.9
siRNA-mediated gene silencing
Cells (2 × 106) growing in log-phase were transfected with 50 pmol of synthetic small interfering RNA (siRNA) duplexes (Qiagen, Hilden, Germany) using Nucleofector technology (Lonza, Cologne, Germany) according to the manufacturer’s instructions. The respective target sequences for both siRNAs targeting STAT3 and the nonsilencing control siRNA (siNEG) are displayed in Supporting Information Table S1.
Establishment of stable single-cell clone populations
Individual Expression Arrest™ TRIPZ lentiviral short-hairpin RNA (shRNA) constructs (Thermo Fisher Scientific, Huntsville, AL) were transfected using Nucleofector technology (Lonza, Cologne, Germany) as previously described.12 The respective target sequences for both shRNAmirs targeting STAT3 and the nonsilencing control shRNAmir (shNEG) are displayed in Supporting Information Table S1. Following selection with 0.8 μg/ml puromycin (Roth, Karlsruhe, Germany), stable single-cell clone (scc) populations were subsequently expanded. Plasmid expression was induced with 1 μg/ml doxycycline (Sigma-Aldrich, Steinheim, Germany), and TurboRFP expression was monitored 96 hr later using fluorescence-activated cell sorting (FACS).
Small molecule-mediated STAT3 inhibition
The nonpeptidic small-molecule inhibitor STATTIC (Santa Cruz Biotechnology, Santa Cruz, CA) was used to inhibit the function of the SH2 domain of STAT3. STATTIC is selective for STAT3 over STAT1 and STAT5, and prevents phosphorylation at Tyr705 and, consequently, dimerization and nuclear translocation.13 Cells were treated with different concentrations of STATTIC for 30 min, followed by a medium change.
RNA isolation and semiquantitative real-time PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, including a DNase treatment step. Subsequently, real-time PCR was performed in a Bio-Rad CFX96 Touch (Bio-Rad, Munich, Germany) as described recently9; the corresponding primer sequences can be found in Supporting Information Table S1. The resulting cycle threshold (Ct) values were normalized according to the mean of three house-keeping genes (i.e. HPRT1, OTUB1 and FBXL12), and the 2–ΔΔCT algorithm14 was applied to estimate the relative gene expression changes between two cell populations.
Western blot analysis
Western blot analysis was performed as previously described.15 Briefly, 20 μg of whole-cell protein lysate was prepared and resolved on a ProGel Tris Glycin 4–12% gel (anamed Elektrophorese, Gross-Bieberau, Germany) or an 8% Bis-Tris-Gel. Proteins were transferred by semidry blotting onto a PVDF membrane (GE Healthcare, Little Chalfont, UK), probed with antibodies by SNAP i.d. technology (Millipore, Billerica, MA) and detected using an ImageQuant LAS 3000 (GE Healthcare, Freiburg, Germany) CCD camera system. The respective antibodies and experimental conditions are listed in Supporting Information Table S2. For evaluation of Interleukin-6 (IL-6) dependent phosphorylation of STAT3, and for confirmation of RNAi- and STATTIC-mediated STAT3 inhibition, cells were serum starved for 24 hr and incubated with 100 ng/ml IL-6 for 30 min before lysis.
Cell Viability Assay
Cellular viability following siRNA-transfection was determined using the CellTiter-Blue® reagent (Promega, Madison, WI). Replicate transfections per siRNA duplex were set up, and reduction of resazurin to resorufin was measured 96 hr after transfection using a plate reader (VICTOR™ X4, Perkin Elmer, Waltham, MA) according to the manufacturer’s instructions. Cellular viability of cells transfected with siRNAs targeting STAT3 was compared to cells transfected with the nonsilencing control siRNA.
Dual luciferase reporter assay
STAT3 transcription factor activity was measured using the Cignal™ Pathway Reporter Assay Kit (Qiagen, Hilden, Germany) and the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI) according to the manufacturers’ instructions. For SW480, wild-type (for inhibitor treatment), siRNA-transfected (72 hr after siRNA transfection) or shRNA-transfected cells (96 hr after induction with doxycycline) were transfected with one microgram of reporter plasmid DNA using 3 μl X-tremeGENE HP DNA Transfection Reagent (Roche, Mannheim, Germany) under serum free conditions. Each sample was cotransfected with a Renilla luciferase reporter plasmid to normalize for transfection efficiency. Twenty-four hours later, wild-type cells were treated with STATTIC for 30 min, and both inhibitor and transfected cells were incubated with 100 ng/ml IL-6 for 90 min and lysed. Subsequently, Firefly and Renilla luciferase activity were measured in a microplate reader (Mithras LB940, Berthold Technologies, Bad Wildbad, Germany). For SW837, wild-type (for inhibitor treatment) or siRNA-transfected cells (48 hr after siRNA transfection) were transfected with 1 μg of reporter plasmid DNA by Nucleofection technology (Lonza, Cologne, Germany). On the next day, wild-type cells were treated with STATTIC for 30 min, and both inhibitor- and transfected cells were subsequently incubated with 100 ng/ml of IL-6 over night and lysed. Relative transcription factor activity was calculated by dividing relative light units (RLU) of the STAT3-specific reporter and the negative control reporter. To determine the STAT3 pathway activation factor, IL-6 stimulated pathway activity was divided by nonstimulated activity.
Chemoradiotherapy and determination of cell survival
Tumor cells growing in log-phase were seeded as single-cell suspensions into 6-well plates and exposed to 3 μM of 5-FU (Sigma-Aldrich, Steinheim, Germany) for 16 hr.9,15 Subsequently, cells were irradiated with a single dose of 1, 2, 4, 6 and 8 Gy of X-rays (Gulmay Medical, Camberley, UK), and a standard colony-forming assay was performed to determine the respective surviving fractions (SF). All colony-forming assays were performed without prior stimulation with IL-6. After defined time periods,9,15 cells were fixed with 70% ethanol and stained. Colonies with more than 50 cells were scored as survivors. Nonirradiated cultures were used for data normalization, and experiments were performed as technical triplicates, independently repeated three times. Survival fractions and dose-modifying factors (DMF) at 37% survival were calculated, and the respective values are shown in Supporting Information Table S3.
Animal model studies
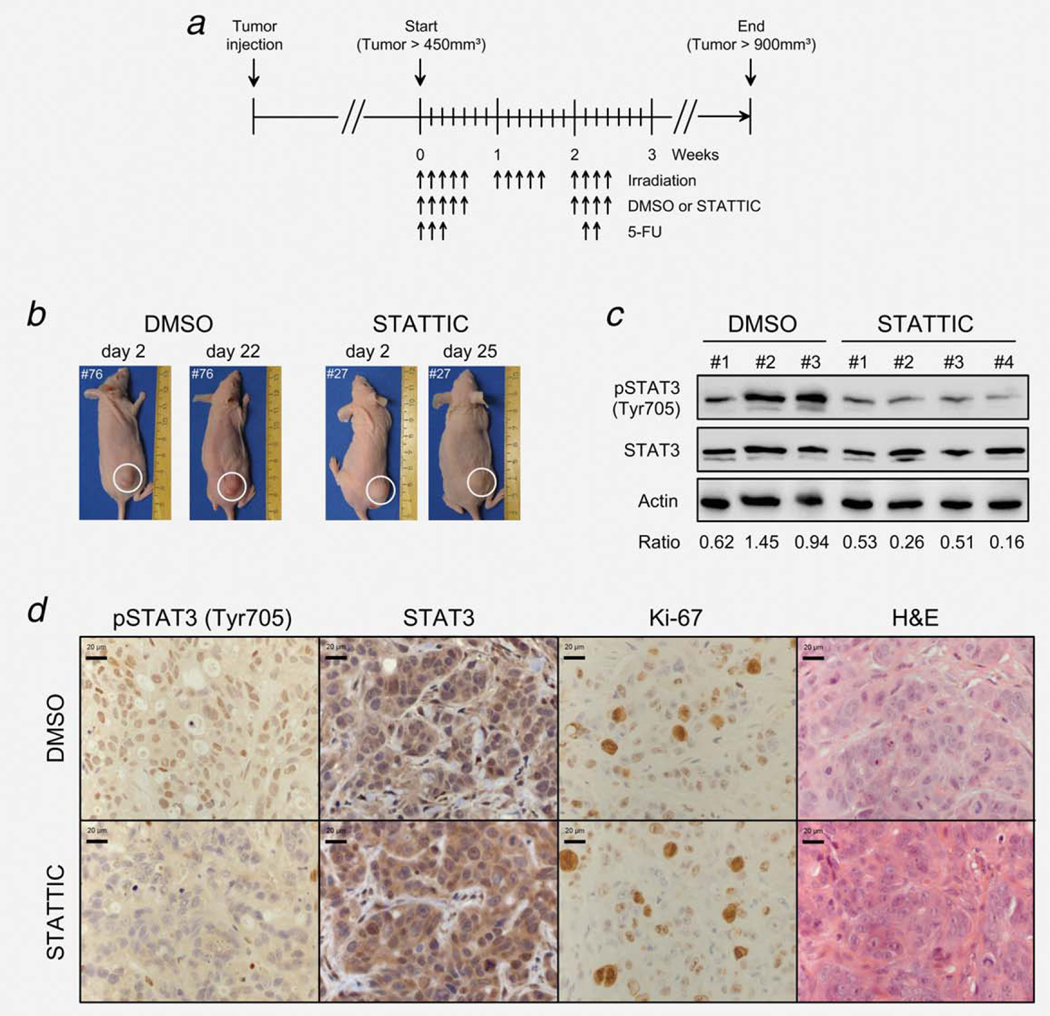
Eight-week-old female athymic nude Naval Medical Research Institute (NMRI)-Foxn1 nu/nu mice were obtained from Janvier (Le Genest St Isle, France) and kept in accordance with the requirements of the German Animal Welfare Act (reference number: G11.559). SW837 cells (2 × 106) were injected subcutaneously into the right flank. Thrice weekly, tumor size was measured to calculate the respective tumor volume using the following formula: volume = (width2 × length)/2.
At a volume of ~450 mm3, either STATTIC (10 mg/kg) or 23% DMSO in sterile PBS was injected subcutaneously into the tumor, as described by Scuto et al.,16 and 5-FU (50 mg/kg) was administered i.p. After 1 hr, nontumor parts were shielded with lead blocks for vital organ protection, and tumors were subsequently irradiated daily with 1.8 Gy for 3 weeks using an X-ray irradiator (Gulmay Medical) operating at 70 kV, 25 mA and with 0.5-mm Al filtration under inhalation anesthesia with Sevoflurane (Abbott, Wiesbaden, Germany). Here, in contrast to the in vitro experiments, we specifically established a treatment protocol that recapitulates clinical conditions, i.e. fractionated doses of both chemotherapy and irradiation.
To document the tumor development, pictures of each mouse were taken once weekly. All experimental groups consisted of ten to twelve mice, including groups with control animals (no CT/RT) consisting of 8–11 mice. In summary, there were four groups: DMSO + CT/RT, STATTIC + CT/RT, DMSO alone (control group 1) and STATTIC alone (control group 2).
Immunohistochemistry
Tumors were fixed in 4% PBS-buffered formaldehyde (Applichem, Darmstadt, Germany) over night and embedded in paraffin. Tissue sections (2 μm) were deparaffinized in 100% xylene and rehydrated through incubation in descending ethanol dilutions (100–60%) followed by boiling at 125 °C for 2 min under pressure (Pascal, Dako, Hamburg, Germany) in different buffers (Citrate pH 6 and EDTA pH 8). To reduce the endogenous peroxidase activity, slides were treated with 3% H2O2 for 10 min and subsequently probed with the primary and secondary antibody. Diaminobenzidine (DAB+, Dako, Hamburg, Germany) was used as chromogen. Antibodies, dilutions and experimental conditions are displayed in Supporting Information Table S2. Tissue sections were imaged using an Axioscop microscope and ZEN software (Carl Zeiss, Jena, Germany).
Statistical analysis
For the in vitro experiments, CT/RT-sensitivity of treated and untreated cells was compared using a multiple linear regression model to describe the normalized SF as dependent variable, given the independent variables of irradiation dose, group (negative control vs. STAT3 inhibition) and replicate pairing. Two different models were computed and compared using analysis of variance (ANOVA). A model including only irradiation dose and replicate was compared to a model that includes additional effects for treatment and a treatment/irradiation dose interaction term. A significant outcome suggests an influence of the STATTIC treatment on the dose response.
For the in vivo experiments, the influence of the different parameters on the tumor volume during CT/RT was assessed using a linear mixed effects model. Tumor volume was modeled as being dependent on the weight of the mouse, the type of treatment (DMSO vs. STATTIC), the day during CT/RT (days 1–19) and a treatment/day interaction term. The individual effect for each mouse was added to the model as random effect. Altogether, ten DMSO-treated and 12 STATTIC-treated mice were included.
To assess the survival time after treatment, Kaplan–Meier analysis was performed. Survival time was computed from the first day of treatment. All mice died at the end of the experiment and, therefore, there was no censored data. Significance between the two treatment groups (DMSO vs. STATTIC) was computed using the log-rank test. The hazard ratio was assessed using a Cox proportional hazards model.
P-Values of less than 0.05 were considered significant. All analyses were performed using the R statistical computing software R (version 2.9.2) and using the R packages nlme and survival. For visualization, data are presented as mean and standard error of the mean (SEM) from at least three independent experiments using the Software Microsoft Excel and Grapher (version 8.2.460). Levels of significance from real-time RT-PCR and luciferase reporter assay experiments were calculated using an unpaired two-tailed Student’s t-test.
Results
STAT3 expression correlates with resistance to CT/RT
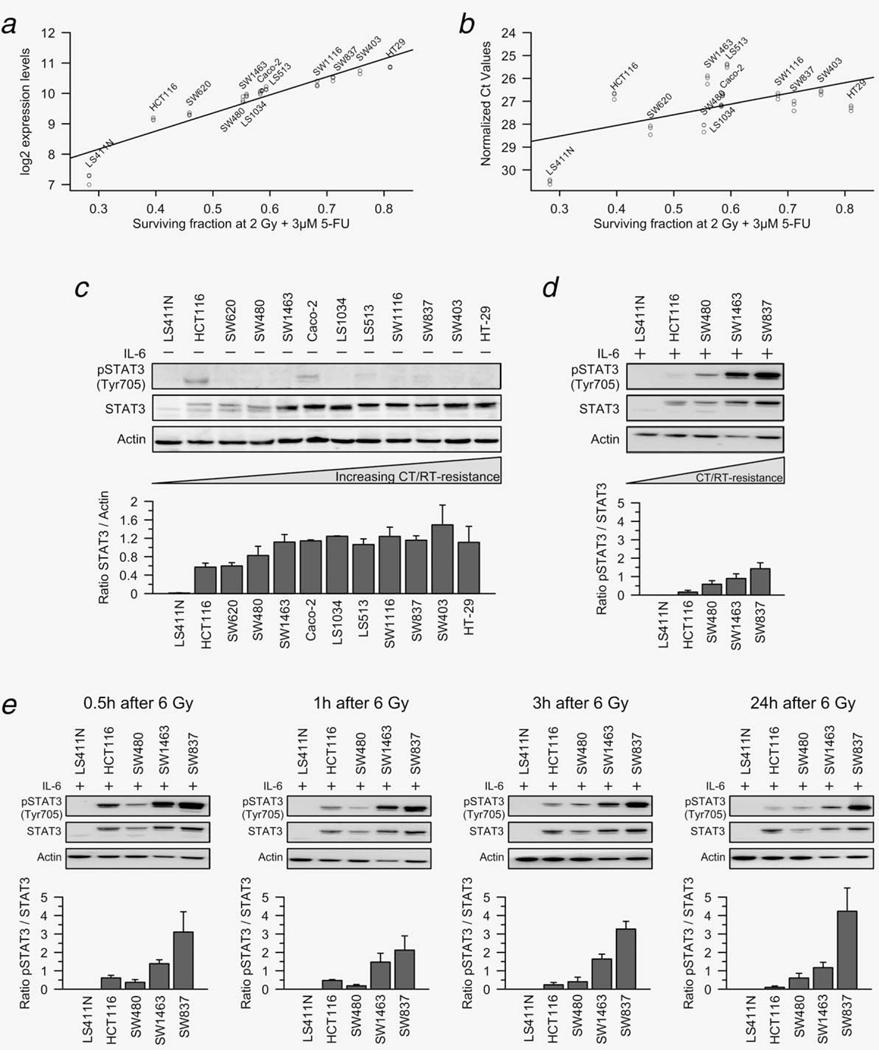
Using microarray-based expression profiling, we have recently established a gene expression signature for CT/RT-sensitivity of CRC cell lines.9 This approach unveiled that STAT3 expression was positively correlated with increasing resistance to CT/RT (Fig. 1a) and uncovered STAT3 as a potential key mediator that renders CRC cells resistant to CT/RT. To directly confirm and extend these findings, we have now used semiquantitative real-time PCR and Western blot analysis. Monitoring of the STAT3 mRNA (Fig. 1b) and STAT3 protein itself (Fig. 1c) showed a positive correlation between STAT3 expression and resistance to CT/RT at both the level of STAT3 transcription and STAT3 translation. In one cell line, i.e. LS411N, there was virtually no STAT3 expression, neither at the mRNA nor protein level.
Figure 1.
STAT3 expression and IL-6-induced phosphorylation of STAT3 correlate with resistance to chemoradiotherapy. (a and b) STAT3 mRNA expression levels correlated positively with increasing resistance of 12 CRC cell lines to CT/RT, measured by microarrays (a, data modified from reference 9) and RT-PCR (b). (c) Western blot analysis confirmed this positive correlation for total STAT3 protein expression, but there was no pSTAT3Tyr705 expression in 10 of 12 cell lines. In HCT116 and Caco-2, very low expression levels of pSTAT3Tyr705 were detected. (d) After incubation with 100 ng/ml IL-6 for 30 min, pSTAT3Tyr705 levels were remarkably higher in chemoradiotherapy-resistant cell lines. (e) When cell lines were exposed to 6 Gy of X-rays, followed by stimulation with 100 ng/ml IL-6 0.5, 1, 3 and 24 hr after irradiation, we again detected remarkably higher levels of phosphorylated STAT3 in chemoradiotherapy-resistant cell lines. This difference remained stable over time. All experiments were done in triplicate and showed similar results (exemplified is one representative experiment with the respective densitometry data ± SEM (pSTAT3/STAT3 ratios) of all replicates).
Chronic STAT3 signaling has been frequently reported in both primary tumors and tumor cell lines, and persistent activation is considered necessary for colon cancer progression.17–21 In ten of 12 CRC cell lines, however, no phosphorylation of STAT3 at the activating tyrosine residue 705 could be detected by immunoblotting with a phosphorylation-site-specific antibody (anti-pSTAT3Tyr705, Fig. 1c). In two cell lines, i.e. HCT116 and Caco-2, very low expression levels of pSTAT3Tyr705 were measured. Hence, STAT3 was not constitutively active in most of these cell lines, consistent with published results.19,22 This prompted us to investigate whether irradiation alone induces the expression and/or phosphorylation of STAT3. As shown in Supporting Information Figure S1, there were no detectable levels of pSTAT3Tyr705 following exposure to 6 Gy of X-rays, suggesting that irradiation-induced DNA-damage does not lead to activation of STAT3 signaling.
These data support the hypothesis that STAT3 plays an important role in conferring resistance of CRC cells to CT/RT. However, activation of STAT3 appears to require additional stimuli.
IL-6-induced STAT3 phosphorylation correlates with resistance to CT/RT
Because STAT3 phosphorylation can be induced by cytokine stimulation, particularly via IL-6,23–25 we measured pSTAT3-Tyr705 levels 30 min after incubation with 100 ng/ml IL-6 in five cell lines with different sensitivities to CT/RT. Importantly, we now detected remarkably higher levels of phosphorylated STAT3 in CT/RT-resistant cell lines. While there were no apparent pSTAT3Tyr705 levels in LS411N and HCT116, which are highly sensitive to CT/RT, SW1463 and SW837, both highly CT/RT-resistant, showed high pSTAT3Tyr705 levels (Fig. 1d).
A similar result was observed when we analyzed whether IL-6-induced phosphorylation of STAT3 persisted following irradiation. Towards this goal, these five cell lines were exposed to 6 Gy of X-rays and subsequently incubated with 100 ng/ml IL-6 for 30 min, 0.5, 1, 3 and 24 hr after irradiation. Again, we detected remarkably higher levels of phosphorylated STAT3 in CT/RT-resistant cell lines (Fig. 1e). This difference remained stable over time.
These data suggest that while IL-6-stimulation is necessary to induce STAT3 phosphorylation, expression levels of total STAT3 correlate positively with increasing resistance to CT/RT.
siRNA-mediated silencing of STAT3 sensitizes to CT/RT
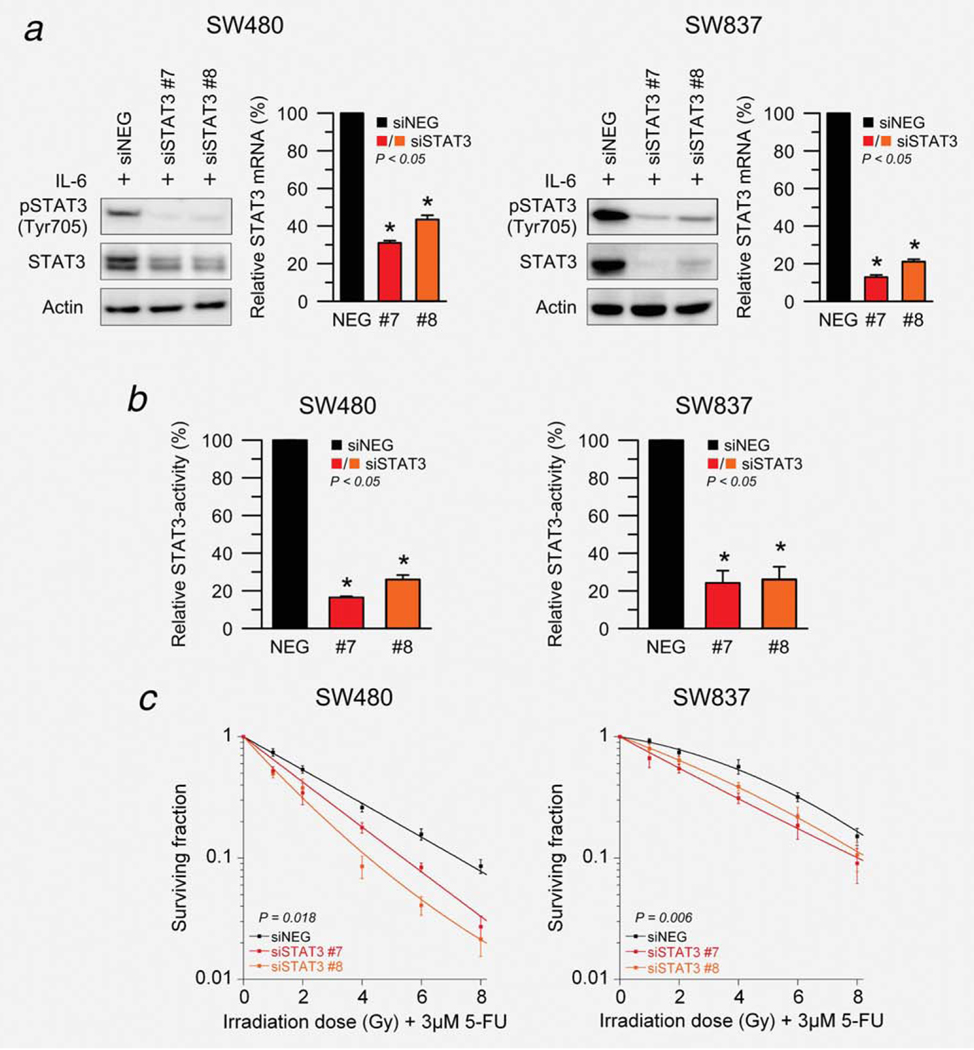
To test whether the observed increased expression of STAT3 in resistant cell lines is of functional relevance for mediating resistance to CT/RT, STAT3 was silenced in SW480 and SW837 using two different siRNAs. These two cell lines were chosen specifically because they show medium (SW480) and high (SW837) resistance to CT/RT,9 and because they are characterized by the typical pattern of chromosomal and transcriptional aberrations that defines primary CRCs.26–28
Successful RNAi-mediated silencing of STAT3 was confirmed 72 hr after transfection using semiquantitative real-time RT-PCR and 96 hr after transfection using Western blot analysis (Fig. 2a). In order to measure protein levels of both pSTAT3Tyr750 and total STAT3, cells were stimulated with 100 ng/ml IL-6 before lysis. Of note, siRNA-mediated silencing of STAT3 affected the viability of these cell lines only slightly (Supporting Information Fig. S2). Because STAT3 is a transcription factor, we also tested its transcription factor activity using a dual luciferase reporter assay. As shown in Figure 2b, transfection with both siRNAs led to a significantly reduced STAT3 reporter activity in SW480 and SW837.
Figure 2.
siRNA-mediated silencing of STAT3 results in a significant sensitization to chemoradiotherapy. (a) Total STAT3 and pSTAT3Tyr705 protein levels decreased 96 hr after transfection (and stimulation with 100 ng/ml IL-6 for 30 min) with two different siRNAs targeting STAT3 compared to a nonspecific negative-control (siNEG) in SW480 (left) and SW837 (right). STAT3 mRNA silencing was established 72 hr after transfection using RT-PCR (P < 0.05). (b) STAT3 transcriptional activity was determined 96 hr after transfection (and stimulation with 100 ng/ml IL-6 for 30 min) using a dual luciferase reporter assay in SW480 (left) and SW837 (right). A negative reporter plasmid served as control. Silencing of STAT3 led to a significant reduction of STAT3 transcription factor activity (P < 0.05). (c) Seventy-two hours after transfection, SW480 (left) and SW837 cells (right) were preincubated with 3 μM of 5-FU for 16 hr, and subsequently irradiated at 1, 2, 4, 6 and 8 Gy of X-rays (without stimulation with IL-6). Silencing of STAT3 significantly increased the sensitivity of both SW480 (P = 0.018; ANOVA model) and SW837 (P = 0.006) to 5-FU-based chemoradiotherapy. Each experiment was repeated three times. Data are displayed as mean values, n = 3, error bars ± SEM.
Finally, both cell lines were treated with 3 μM of 5-FU over night, and subsequently irradiated at 1, 2, 4, 6 and 8 Gy of X-rays 96 hr after siRNA transfection (without stimulation with IL-6). Importantly, silencing of STAT3 significantly increased the sensitivity of both SW480 (P = 0.018; siSTAT3 effect from ANOVA model) and SW837 (P = 0.006) to 5-FU-based CT/RT (Fig. 2c).
STATTIC-mediated inhibition of STAT3 sensitizes to CT/RT
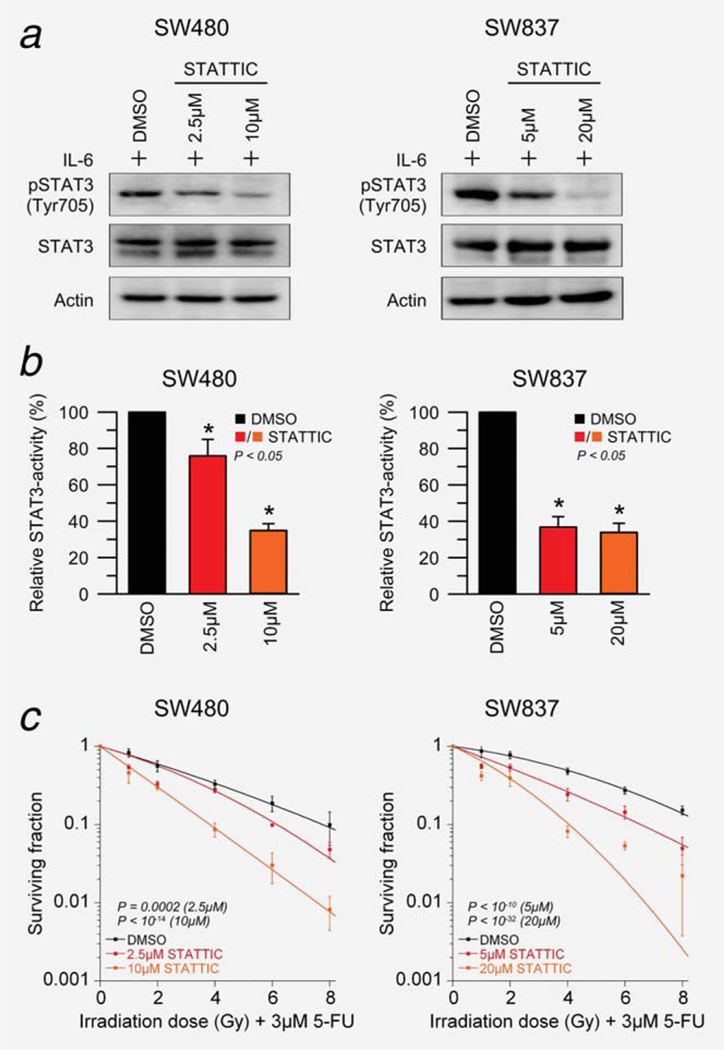
To independently validate this observed effect, SW480 and SW837 cells were treated with two doses of a small-molecule inhibitor of STAT3. STATTIC inhibits the function of the SH2 domain of STAT3 in a dose-dependent manner (Supporting Information Fig. S3), preventing phosphorylation at Tyr705 and, subsequently, dimerization and nuclear translocation.13 Successful inhibition of STAT3 phosphorylation was confirmed 1 hr after STATTIC-treatment and stimulation with 100 ng/ml IL-6 using Western blot analysis (Fig. 3a), and decreased STAT3 activity was established using a dual luciferase reporter assay (Fig. 3b).
Figure 3.
Small molecule-mediated inhibition of pSTAT3Tyr705 sensitizes SW480 and SW837 cells to chemoradiotherapy in a dose-dependent manner. (a) SW480 (left) and SW837 cells (right) were treated with DMSO or different doses of STATTIC for 30 min (SW480: 2.5 and 10 μM; SW837: 5 and 20 μM, respectively), and successful inhibition of pSTAT3Tyr705 was confirmed after IL-6 stimulation (100 ng/ml) for 30 min using Western blot analysis. (b) STAT3 reporter plasmid or control reporter plasmid transfected cells were treated with DMSO or STATTIC, and a dual luciferase reporter assay demonstrated, after stimulation with 100 ng/ml IL-6 (SW480: 90 min; SW837: over night), decreased STAT3 activity following STAT3 inhibitor treatment in both cell lines (P < 0.05). (c) Cell lines were treated with DMSO or STATTIC, incubated with 3 μM of 5-FU over night and subsequently irradiated at 1, 2, 4, 6 and 8 Gy of X-rays (without stimulation with IL-6). STATTIC treatment resulted in a significant sensitization to chemoradiotherapy in a dose-dependent manner in both SW480 (2.5 μM, P = 0.0002; 10 μM, P < 10−14; ANOVA model) and SW837 (5 μM, P < 10−10; 20 μM, P < 10−32). Experiments were done in triplicates, error bars ± SEM.
Subsequently, SW480 and SW837 cells were treated with DMSO or different concentrations of STATTIC, incubated with 3 μM of 5-FU over night, followed by irradiation at 1, 2, 4, 6 and 8 Gy of X-rays (without stimulation with IL-6). As shown in Figure 3c, STATTIC treatment resulted in a significant sensitization to CT/RT in a dose-dependent manner in both SW480 (2.5 μM, P = 0.0002; 10 μM, P < 10−14; inhibitor effect from ANOVA model) and SW837 (5 μM, < 10−10; 20 μM, P < 10−32).
These results suggest that sensitization to CT/RT following STAT3 inhibition is, at least in part, dependent on phosphorylation.
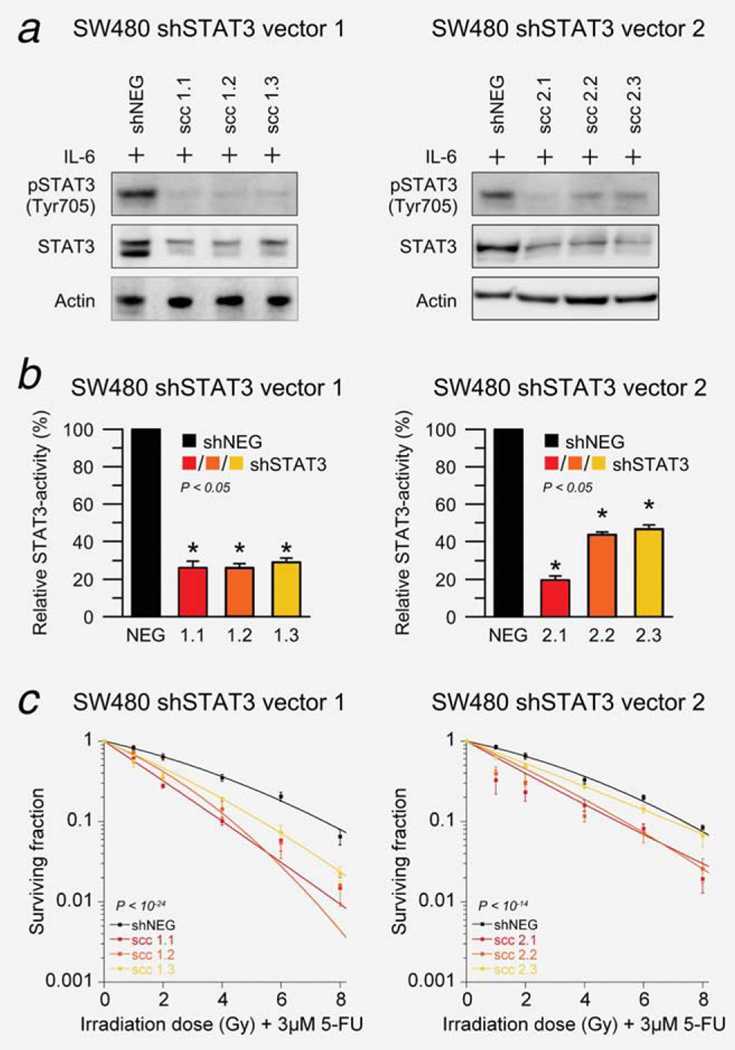
shRNA-mediated silencing of STAT3 sensitizes to CT/RT
Because the RNAi machinery can also be harnessed to stably suppress gene expression, we aimed to independently reproduce our siRNA results using shRNA expression cassettes that can be induced to express shRNAmirs targeting STAT3. Stable single-cell clone populations were established, and three clones from each vector were selected for further experimentation. Successful silencing of total STAT3 and pSTAT3Tyr705 was demonstrated by Western blot analysis 120 hr after doxycycline induction and stimulation with 100 ng/ml IL-6 (Fig. 4a) and reduced STAT3 transcription factor activity by a dual luciferase assay (Fig. 4b). The inducibility of these clones, confirmed by FACS analysis, is displayed in Supporting Information Figure S4. Of note, expression of the respective shRNA constructs did not result in obvious morphological cellular changes (data not shown). Importantly, shRNA-mediated silencing of STAT3 led to a significantly increased treatment sensitivity (vector 1, P < 10−24; vector 2, P < 10−14; shSTAT3 effect from ANOVA model), as shown in Figure 4c.
Figure 4.
shRNA-mediated silencing of STAT3 sensitizes SW480 cells to chemoradiotherapy. (a) Stable single-cell clone populations were established for each inducible shRNA vector targeting STAT3, and successful silencing was confirmed 120 hr after addition of 1 μg/ml doxycycline to induce shRNA expression and stimulation with 100 ng/ml IL-6 for 30 min using Western blotting. (b) Reduced STAT3 transcription factor activity was demonstrated using a dual luciferase reporter assay (P < 0.05). (c) Ninety-six hours after doxycycline induction, cell clones were incubated with 3 μM of 5-FU and irradiated at 1, 2, 4, 6 and 8 Gy of X-rays (without stimulation with IL-6). shRNA-mediated silencing of STAT3 led to a significantly increased treatment sensitivity (vector 1, P < 10−24; vector 2, P < 10−14; ANOVA model). All experiments were done as triplicates and showed similar results, error bars ± SEM.
STAT3 inhibition leads to chemoradiotherapy-sensitization in a subcutaneous xenograft model
Next, we aimed to assess the effect of STAT3 inhibition on treatment sensitivity in vivo. Towards this goal, we first established a treatment protocol that recapitulates clinical conditions and mimics the real treatment situation, i.e. fractionated doses of both chemotherapy and irradiation (Fig. 5a).
Figure 5.
Establishment of a subcutaneous xenograft model that recapitulates clinical conditions, i.e. fractionated doses of both chemotherapy and irradiation. (a) Two million SW837 cells (100 μl) were injected subcutaneously into the right flank of NMRI-Foxn1 nude mice. At a volume of ~450 mm3, mice were separated into two groups, DMSO (n = 10) and STATTIC (n = 12) and treated as indicated (black arrows pointing upward) at irradiation doses of 1.8 Gy (14×), intraperitoneal injections of 50 mg/kg 5-FU (5×) and intratumoral injections of 23% DMSO or 10 mg/kg STATTIC (9×). (b) To document tumor development, pictures of each mouse were taken once weekly. (c) To confirm inhibition of STAT3 phosphorylation in vivo, selected tumor-bearing mice were randomly sacrificed 2 hr after STATTIC or DMSO injection and subjected to Western blot analysis. Compared to DMSO-treated mice, we detected remarkably reduced pSTAT3Tyr705 levels in STATTIC-treated mice. Displayed is a representative blot from three DMSO- and four STATTIC-treated mice with the respective densitometry data (pSTAT3/STAT3 ratios). (d) Immunohistochemical analysis also confirmed remarkably reduced pSTAT3Tyr705 levels in STATTIC-treated mice compared to DMSO-treated mice (exemplified is one representative DMSO- and STATTIC-treated tumor, randomly selected). STATTIC- and DMSO-treated tumors showed similar rates of total STAT3 expression, proliferation (Ki-67 staining) and cellular morphologies (H&E staining).
Two million SW837 cells were injected subcutaneously into the right flank of nude mice, and tumor size was documented (Fig. 5b) and measured thrice weekly to calculate the respective tumor volume. To confirm inhibition of STAT3 phosphorylation in vivo, selected tumor-bearing mice were randomly sacrificed 2 hr after STATTIC (10 mg/kg) or 23% DMSO injection and subjected to Western blot (Fig. 5c) and immunohistochemical analysis (Fig. 5d). Compared to DMSO-treated mice, we detected remarkably reduced pSTAT3Tyr705 levels in STATTIC-treated mice (Figs. 5c and 5d). Ki-67 and H&E staining confirmed vital and proliferating cells in the respective sections of the tumors (Fig. 5d).
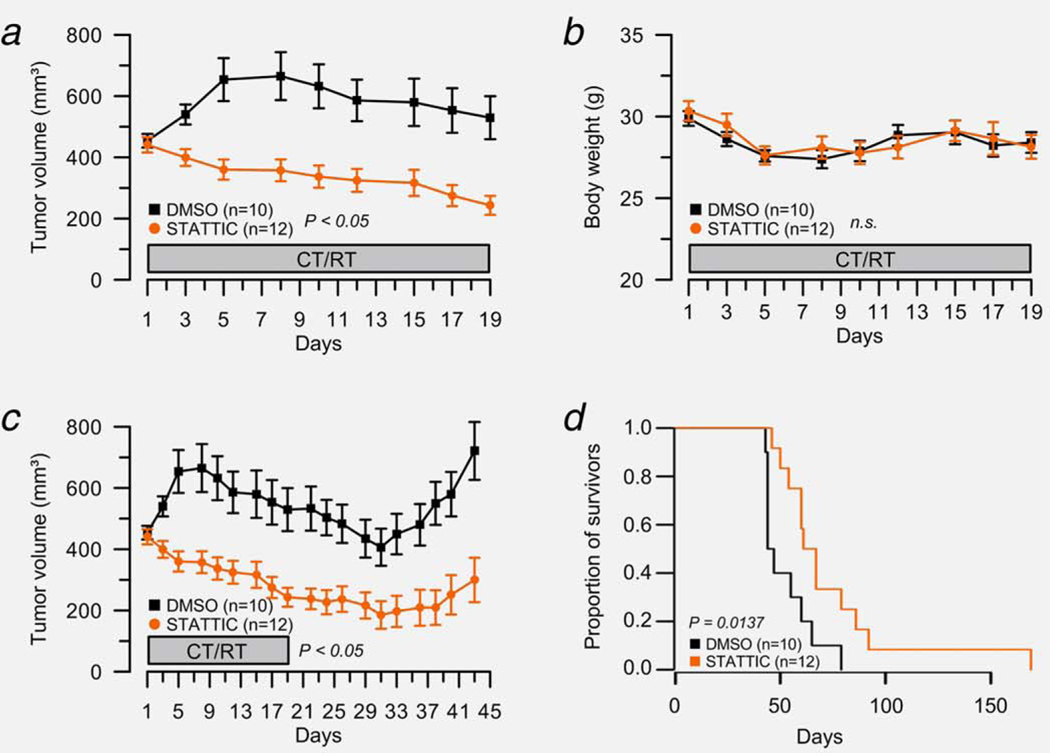
As displayed in Figure 6a, the tumor volume of DMSO-treated mice (n = 10) remained relatively stable during the administration of 5-FU (50 mg/kg) and irradiation (1.8 Gy/day). In clear contrast, the tumor volume of STATTIC-treated mice (n = 12) decreased over time. To demonstrate whether the observed differences in tumor volumes between the groups were statistically significant, we fitted a linear mixed effects model to the data. The model takes into account not only the treatment group, but also the individual weight of the mice, the days of treatment during CT/RT and the possible variation between the different mice as random effects. The model estimates the expected tumor volume to be 833 mm3. Overall, there was a slight increase of tumor volume by treatment day (0.76, P = 0.63), but not statistically significant. However, mice in the STATTIC group overall exhibited a lower tumor volume (−132.7, P = 0.046), and the volume was further decreased with each treatment day (−10.3, P = 2.8e–6). Therefore, we conclude that there was a statistically significant effect of the STATTIC treatment on the reduction of tumor volume during the CT/RT treatment. The body weight of the respective mice was not significantly different between both groups (Fig. 6b). Control mice that were only treated with either inhibitor or DMSO, but without CT/RT, showed no significant tumor growth inhibition (Supporting Information Fig. S5).
Figure 6.
STAT3 inhibition sensitizes SW837 cells to chemoradiotherapy in vivo. (a) Tumor size was measured thrice weekly to calculate the respective tumor volume during the administration of 5-FU and radiation therapy. While the tumor volume of DMSO-treated mice (n = 10) remained relatively stable, the tumor volume of STATTIC-treated mice (n = 12) decreased significantly over time (P = 0.046, linear mixed effects model). (b) With respect to the body weight, there was no significant difference between both groups. (c) All mice were monitored until the tumor volume exceeded 900 mm3 (start volume ×2). Tumor regrowth was significantly delayed in STATTIC-treated mice compared with control animals (P = 2.8 10−6, linear mixed effects model). (d) Kaplan–Meier curves were calculated to evaluate a potential survival benefit for mice under STATTIC treatment. The median survival for the DMSO group was 45.5 days, compared to 64.0 days for the STATTIC group, indicating a clear survival benefit for STATTIC-treated mice (P = 0.0137; hazard ratio = 0.33, 95% confidence interval: 0.13–0.83).
Finally, we assessed tumor regrowth characteristics. To this end, all mice were monitored until the tumor volume exceeded 900 mm3 (start volume ×2). Again, tumor regrowth was significantly delayed in STATTIC-treated mice compared with control animals (Fig. 6c; P = 2.8 10−6, treatment effect from linear mixed effects model). Finally, we evaluated whether there was also a significant survival benefit for mice under STATTIC treatment. The Kaplan–Meier curves showed a clear survival benefit for STATTIC-treated mice (Fig. 6d). The median survival for the DMSO group was 45.5 days, compared to 64.0 days for the STATTIC group. This difference was statistically significant in the log-rank test (P = 0.0137). In a Cox proportional hazards model, the hazard ratio between the groups was estimated to be 0.33 (95% confidence interval: 0.13–0.83), indicating that STATTIC-treated mice have a better survival.
Collectively, these results demonstrate that inhibition of STAT3 is associated with a significant sensitization of a priori resistant CRC cells to CT/RT in vitro and in vivo.
Discussion
A considerable percentage of primary rectal cancers are resistant to preoperative 5-FU-based CT/RT, which constitutes the standard treatment in advanced stages of this disease.10,11 This represents a major clinical problem, because it exposes these patients to the potential side effects of both irradiation and chemotherapy without a clear benefit. It is therefore exceedingly important to determine the molecular characteristics underlying this resistance and to identify novel molecular targets that can be manipulated to sensitize a priori resistant tumors. Our results identify STAT3 as such a molecular target in rectal cancer.
Persistent and aberrant activation of STAT signaling is a recurrent feature of human malignancies.1,2,5–8 In CRC, specifically, STAT3 signaling is frequently activated in both primary tumors and cell lines,17–22 while constitutively activating STAT3 mutations are rare events.29 Corvinus et al. were the first to demonstrate that aberrant STAT3 activity is associated with enhanced proliferation and tumor growth of colon cancer cell lines.19 Interestingly, the authors also reported consistent loss of constitutive activation upon cultivation, which could be reactivated by cytokine stimulation, particularly via IL-6.19 Similarly, Lin et al. showed that blockade of STAT3 signaling significantly decreased the viability of colon cancer cell lines due to apoptosis and cell-cycle arrest.17 Subsequently, it was demonstrated that STAT3 enhances the invasive growth of colon cancer cell lines through an increased expression of matrix metalloproteinases,30 probably as a result of protein–protein interactions with AP-1.31 The activation of STAT3 signaling has also been linked to various other pathophysiologies, including chronic inflammation in mouse models of colitis-associated cancers.32–34 Only recently, growing evidence suggested that STAT3 signaling is highly activated in putative colon cancer-initiating cells and that it is required for their proliferation and survival.35,36 However, STAT3 signaling has not been previously associated with resistance to CT/RT in CRC37; therefore, our results are novel.
In contrast, recent findings from other malignancies imply a role for STAT3 signaling in mediating radiation resistance. Kim et al. were the first to demonstrate that inhibition of STAT3 resulted in a radiosensitization of breast cancer cells.38 Similar findings have been subsequently reported for A431 squamous cell carcinomas cells,39 glioma cells,40 head and neck squamous cell carcinoma cells41 and glioblastoma tumor-initiating cells.42 Our results add weight to the growing body of evidence that STAT3 inhibition may represent a potential strategy for sensitizing radiation-resistant tumors in the clinical setting.
The tumor-promoting features of STAT3 signaling are mediated by two mechanisms: first, directly, through an activation of the tumor cells, i.e. increased tumor cell proliferation and neo-angiogenesis (cancer cell-intrinsic pathway); second, indirectly, through a suppression of antitumor responses of the tumor-associated stroma and stimulation of tumor-promoting inflammation (cancer cell-extrinsic pathway).5–7 Because the tumor microenvironment itself emerges more and more as an attractive therapeutic target in its own right,43,44 the “combinatorial” strategy of targeting both a cancer cell-intrinsic and cancer cell-extrinsic pathway simultaneously holds considerable promise for future treatment concepts.8,45 For instance, it has been demonstrated that STAT3 activation in immune cells plays a critical role in tumor immune evasion, and STAT3 inhibition induces T cell- and natural killer cell-dependent growth inhibition in vivo.46,47 In addition, targeting STAT3 in the myeloid compartment increased killing activity and tumor infiltration of adoptively transferred T cells.48 In this context, it is becoming increasingly clear that, after preoperative CT/RT, the presence of residual positive lymph nodes, an integral part of the immune system, is one of the strongest predictors for poor disease-free survival in patients with rectal cancer.49,50
While our findings of STAT3-dependent resistance to CT/RT are novel and of obvious pathophysiological relevance, relevant details remain to be resolved: first, the precise cellular mechanisms through which STAT3 signaling mediates CT/RT-resistance remain to be deciphered. Second, our in vivo study is limited by the fact that STATTIC was administered locally into the tumor. However, in the absence of CT/RT, we observed no difference in growth characteristics between STATTIC- and DMSO-treated mice. It is therefore unlikely that the intratumoral injections alone explain the increased sensitivity to CT/RT. And third, as STATTIC is not a favorable candidate therapeutic, other agents with greater potency and specificity such as the novel JAK inhibitors need to be evaluated for potential clinical application.
In summary, we provide first evidence that STAT3 mediates resistance of CRC cell lines to CT/RT and that inhibition of STAT3 signaling may represent a promising strategy to sensitize resistant rectal cancers to irradiation. Because expression of phosphorylated STAT3 can be detected in ~30–40% of rectal cancers,18,20,21 STAT3 inhibition could play a prominent role in future treatment concepts for patients with this disease.
Supplementary Material
What’s new?
A considerable percentage of rectal cancers are resistant to preoperative chemoradiotherapy, which exposes patients to the potential side effects of both irradiation and chemotherapy without clear benefits. In this study, IL-6-stimulated expression levels of phosphorylated STAT3 were remarkably higher in chemoradiotherapy-resistant colorectal cancer cell lines. RNAi- and small molecule-mediated STAT3 inhibition sensitized to chemoradiotherapy in vitro in a dose-dependent manner, which led to a profound chemoradiotherapy-sensitization in a subcutaneous xenograft model. These results highlight a potential role of STAT3 in treatment resistance, and provide first proof of concept that STAT3 represents a promising novel molecular target for sensitizing resistant rectal cancers to chemoradiotherapy.
Acknowledgements
The authors are grateful to Jessica Eggert, Stefanie Müller, Chang-Rong Lai and Carolin Herzberg for excellent technical support. They also thank the members of the central animal research facility for their continues support, Matthias Dobbelstein and Holger Bastians for helpful discussions and Michael Klintschar for short-tandem repeat analyses. Materials and data from this manuscript are part of the doctoral theses of Birte Roesler and Christian Bielfeld.
Grant sponsor: Deutsche Forschungsgemeinschaft
Grant numbers KFO 179: GR 3376/1–1, GR 3376/1–2
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 2004;4: 97–105. [DOI] [PubMed] [Google Scholar]
- 2.Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2005;2: 315–24. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell 1999;98:295–303. [DOI] [PubMed] [Google Scholar]
- 4.Darnell JE Jr., STATs and gene regulation. Science 1997;277:1630–5. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7:41–51. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012;30:1005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzner M, Emons G, Kramer F, et al. A gene expression signature for chemoradiosensitivity of colorectal cancer cells. Int J Radiat Oncol Biol Phys 2010;78:1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030–47. [DOI] [PubMed] [Google Scholar]
- 11.Rodel C, Hofheinz R, Liersch T. Rectal cancer: state of the art in 2012. Curr Opin Oncol 2012;24: 441–7. [DOI] [PubMed] [Google Scholar]
- 12.Grade M, Hummon AB, Camps J, et al. A genomic strategy for the functional validation of colorectal cancer genes identifies potential therapeutic targets. Int J Cancer 2011;128:1069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schust J, Sperl B, Hollis A, et al. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 2006;13:1235–42. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 15.Kendziorra E, Ahlborn K, Spitzner M, et al. Silencing of the Wnt transcription factor TCF4 sensitizes colorectal cancer cells to (chemo-) radiotherapy. Carcinogenesis 2011;32:1824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scuto A, Kujawski M, Kowolik C, et al. STAT3 inhibition is a therapeutic strategy for ABC-like diffuse large B-cell lymphoma. Cancer Res 2011; 71:3182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Q, Lai R, Chirieac LR, et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol 2005;167: 969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusaba T, Nakayama T, Yamazumi K, et al. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol 2005;58:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corvinus FM, Orth C, Moriggl R, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005;7:545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikawa T, Baba Y, Yamauchi M, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res 2011;17:1452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monnien F, Zaki H, Borg C, et al. Prognostic value of phosphorylated STAT3 in advanced rectal cancer: a study from 104 French patients included in the EORTC 22921 trial. J Clin Pathol 2010;63:873–8. [DOI] [PubMed] [Google Scholar]
- 22.Klampfer L. The role of signal transducers and activators of transcription in colon cancer. Front Biosci 2008;13:2888–99. [DOI] [PubMed] [Google Scholar]
- 23.Waldner MJ, Foersch S, Neurath MF. Interleukin-6—a key regulator of colorectal cancer development. Int J Biol Sci 2012;8:1248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets 2008;9:369–74. [DOI] [PubMed] [Google Scholar]
- 25.Rose-John S, Mitsuyama K, Matsumoto S, et al. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des 2009;15:2095–103. [DOI] [PubMed] [Google Scholar]
- 26.Grade M, Hormann P, Becker S, et al. Gene expression profiling reveals a massive, aneuploidy-dependent transcriptional deregulation and distinct differences between lymph node-negative and lymph node-positive colon carcinomas. Cancer Res 2007;67:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camps J, Nguyen QT, Padilla-Nash HM, et al. Integrative genomics reveals mechanisms of copy number alterations responsible for transcriptional deregulation in colorectal cancer. Genes Chromosomes Cancer 2009;48:1002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camps J, Pitt JJ, Emons G, et al. Genetic amplication of the NOTCH modulator LNX2 upregulates the WNT/beta-catenin pathway in colorectal cancer. Cancer Res 2013;73:2003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Network CGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsareva SA, Moriggl R, Corvinus FM, et al. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia 2007;9:279–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zugowski C, Lieder F, Muller A, et al. STAT3 controls matrix metalloproteinase-1 expression in colon carcinoma cells by both direct and AP-1-mediated interaction with the MMP-1 promoter. Biol Chem 2011;392:449–59. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med 2001;193:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009;15:91–102. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009;15:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin L, Liu Y, Li H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer 2011;105:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Liu A, Peng Z, et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 2011;71:7226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grade M, Wolff HA, Gaedcke J, et al. The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbecks Arch Surg 2012;397:543–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KW, Mutter RW, Cao C, et al. Inhibition of signal transducer and activator of transcription 3 activity results in down-regulation of Survivin following irradiation. Mol Cancer Ther 2006;5:2659–65. [DOI] [PubMed] [Google Scholar]
- 39.Bonner JA, Trummell HQ, Willey CD, et al. Inhibition of STAT-3 results in radiosensitization of human squamous cell carcinoma. Radiother Oncol 2009;92:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao L, Li F, Dong B, et al. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys 2010;77:1223–31. [DOI] [PubMed] [Google Scholar]
- 41.Chen YW, Chen KH, Huang PI, et al. Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma—derived CD44(1)ALDH1(1) cells. Mol Cancer Ther 2010;9:2879–92. [DOI] [PubMed] [Google Scholar]
- 42.Yang YP, Chang YL, Huang PI, et al. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J Cell Physiol 2012;227:976–93. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22. [DOI] [PubMed] [Google Scholar]
- 44.Swartz MA, Iida N, Roberts EW, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res 2012;72:2473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: rationality and rationale design of inhibitors. Expert Opin Investig Drugs 2011;20:1263–75. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004;10:48–54. [DOI] [PubMed] [Google Scholar]
- 47.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005;11:1314–21. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann A, Kortylewski M, Kujawski M, et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res 2010;70:7455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishihara S, Watanabe T, Kiyomatsu T, et al. Prognostic significance of response to preoperative radiotherapy, lymph node metastasis, and CEA level in patients undergoing total mesorectal excision of rectal cancer. Int J Colorectal Dis 2010;25:1417–25. [DOI] [PubMed] [Google Scholar]
- 50.Chang GJ, Rodriguez-Bigas MA, Eng C, et al. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer 2009;115:5432–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.