Abstract
Background:
Low-gradient aortic stenosis (LG-AS) is characterized by the combination of an aortic valve area compatible with severe stenosis and a low transvalvular mean gradient with low-flow state (i.e., indexed stroke volume <35 mL/m2) in the presence of reduced (classical low-flow AS) or preserved (paradoxical low-flow AS) ejection fraction. Furthermore, the occurrence of a normal-flow LG-AS is still advocated by many authors. Within this diagnostic complexity, the diagnosis of severe AS remains challenging.
Objective:
The general objective of the Discordant Echocardiographic Grading in Low-gradient AS (DEGAS Study) study will be to assess the prevalence of true severe AS in this population and validate new parameters to improve the assessment and the clinical decision-making in patients with LG-AS.
Methods and Analyses:
The DEGAS Study of the Italian Society of Echocardiography and Cardiovascular Imaging is a prospective, multicenter, observational diagnostic study that will enroll consecutively adult patients with LG-AS over 2 years. AS severity will be ideally confirmed by a multimodality approach, but only the quantification of calcium score by multidetector computed tomography will be mandatory. The primary clinical outcome variable will be 12-month all-cause mortality. The secondary outcome variables will be (i) 30-day mortality (for patients treated by Surgical aortic valve replacement or TAVR); (ii) 12-month cardiovascular mortality; (iii) 12-month new major cardiovascular events such as myocardial infarction, stroke, vascular complications, and rehospitalization for heart failure; and (iv) composite endpoint of cardiovascular mortality and hospitalization for heart failure. Data collection will take place through a web platform (REDCap), absolutely secure based on current standards concerning the ethical requirements and data integrity.
Keywords: Aortic valve calcium score, aortic valve stenosis, echocardiography, diagnosis, dobutamine stress echocardiography
RATIONALE
Patients with low-gradient aortic stenosis (LG-AS) are among the most challenging encountered in patients with valvular heart disease. The two main goals for optimal risk stratification and therapeutic management of patients with LG-AS are (i) to accurately discriminate severe aortic stenosis (SAS) versus moderate aortic stenosis (MAS) stenosis and (ii) to accurately quantify the severity of myocardial impairment. Unfortunately, the echocardiographic parameters currently used in patients with LG-AS to assess the severity of valvular and myocardial dysfunction are far from being optimal, and as a consequence, a substantial proportion of these patients is misevaluated and may thus not receive the optimal therapy.
Assessing aortic valve stenosis severity
Low-gradient AS is generally characterized by the combination of an aortic valve area (AVA) compatible with severe stenosis (i.e., ≤1.0 cm2 and ≤0.6 cm2/m2 when indexed for body surface area), a low transvalvular gradient (mean gradient <40 mmHg), with or without a low LV ejection fraction ([EF] ≤50%), and with or without a low-flow state (stroke volume index [SVi] <35 mL/m2). Patients with low EF represent around 5% of the AS population, patients with normal EF but low-flow state 15%, and patients with normal EF and normal flow 20%.[1]
Thus, LG-AS, irrespective of transvalvular flow/EF, is a frequent finding in clinical practice, with up to 40% of patients harboring discrepant results at transthoracic echocardiography examination. In these patients, the diagnostic is challenging since at the outset, it is impossible to distinguish between patients having SAS from those having MAS. Yet, this distinction is essential since patients with SAS will generally benefit from aortic valve replacement (AVR), whereas those with MAS may not necessarily benefit.[2]
Because of the well-known flow-dependency of the mean gradient, the presence of low flow with either a depressed (“classical”) or preserved (“paradoxical”) EF increases the likelihood of a true-SAS. In general, a multimodality imaging approach that includes transthoracic echocardiography, dobutamine stress echocardiography (DSE), and aortic calcium score at multidetector computed tomography (MDCT), together with the verification of the accuracy of the Doppler echocardiographic measurements, is necessary for the appropriate quantification of AS.[3,4]
In the occurrence of normal flow (i.e., SVi ≥35 mL/m2), the presence of a nonsevere AS is still advocated by many authors. However, from a pathophysiological and fluid dynamics standpoint, after excluding measurement errors and the inherent inconsistencies in guidelines of AS severity criteria, the presence of normal-flow low-gradient AS (NF-LG-AS) may be explained by several factors among which the most important and obvious is the presence of a low transvalvular flow rate (flow rate = SV/ejection time), despite a normal SVi that may occur in patients with bradycardia and prolonged LV ejection time.[5] Hence, recent evidence has shown that a significant proportion of patients with NF-LG-AS patients have true severe AS.[5,6]
The other main factor affecting the AVA/gradient relationship and leading to a NF-LG pattern despite the presence of severe AS is abnormal arterial hemodynamics: the presence of systemic hypertension and reduced arterial compliance has been shown to decrease the SVi, prolong LV ejection time, and may also blunt the transvalvular gradients and velocities by the faster and earlier reflection of the arterial wave. Therefore, there is a growing interest in assessing the prognostic value of transvalvular flow rate and valvuloarterial impedance in AS. Finally, diastolic dysfunction[7] and blood biomarkers, such as type-B natriuretic peptide (BNP), are predictive of outcome in patients with AS.[8] Preliminary data on classical LF-LG-AS have shown that both BNP and troponin may improve risk stratification.[9,10] However, larger studies are required to assess their usefulness in LG-AS patients.
Assessing the left ventricular contractile/systolic/pump reserve in low-flow low-gradient pattern by dobutamine stress echocardiography
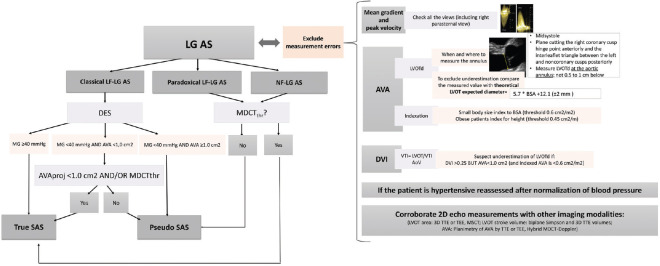
A low-dose (up to 20 μg/kg/min) DSE is useful in patients with low EF and LG-AS to assess the presence of LV contractile reserve and to distinguish SAS versus MAS [Figure 1]. DSE has received a Class IIa (Level of evidence B) recommendation in the ACC/AHA-ESC guidelines for the assessment of these patients.[11,12,13] However, the traditional DSE parameters lack of accuracy in assessing the severity of valvular and myocardial dysfunction. There is, thus, an important need for the development of new more accurate parameters of disease severity in patients with low classical LG-AS. Moreover, DSE is not recommended in patients with paradoxical LG-AS.[11,12,13]
Figure 1.
Usefulness of dobutamine stress echocardiography for clinical decision-making. The “?” indicates the parameters and criteria that needs to be further validated or refined. FR: flow rate (in ml/second); AVA: aortic valve area (in cm2); MG: mean transvalvular gradient (in mmHg); LG-AS: low-gradient severe aortic stenosis (AVA ≤ 1.0 cm2 and MG < 40 mmHg); LF: low-flow (stroke volume index < 35 ml/m2); NF: normal flow (stroke volume index ≥ 35 ml/m2): AVAProj: projected AVA at normal flow rate (in cm2); SAS: severe aortic stenosis; MDCT: multidetector computed tomography; MDCTThr: aortic valve calcium score thresholds measured by MDCT: men > 2000 AU and women > 1200 AU, aortic valve calcium density (i.e., calcium score divided by aortic annulus area) ≥500 AU/cm2 in men, ≥300 AU/cm2 in women)
The assessment of LV “myocardial contractile” reserve (i.e., LV systolic reserve sensu stricto) is an important aspect of the risk stratification process in classical LG-AS Figure 1, because patients with no evidence of contractile reserve, generally defined as a percent increase in SV <20% during DSE, have markedly higher operative mortality (20%–33%) compared to those with contractile reserve (5%–8%).[14,15,16,17] Nevertheless, in the subset of patients with no contractile reserve, the long-term survival is still much lower in patients treated by surgical AVR compared to those treated medically, despite high operative mortality.[17] Furthermore, in the patients with no contractile reserve who survive the operation, the postoperative improvement in EF as well as the late survival rate is as good as in the subset of patients with contractile reserve.[15] Furthermore, after transcatheter AVR, the contractile reserve has no impact.[18] These findings are likely related to the fact that the absence of contractile reserve on DSE is not uniquely determined by the extent of myocardial impairment, per se, but rather by a greater imbalance between the severity of the stenosis and the myocardial reserve resulting in an increased afterload mismatch.[19] Hence, in the context of classical LF-LG-AS, the augmentation of SV during DSE should be more appropriately described as “LV pump” reserve, given that this parameter is not only a marker of intrinsic myocardial impairment but is also influenced by the degree of stenosis severity as well as other factors. However, the impact of this LV pump reserve on the outcome is largely conflictual and requires further assessment. Finally, SV represents only one parameter of the LV pump reserve as opposed to the total flow rate across the aortic valve.[20] Thus, the flow rate could be a better marker than SV and “LV pump” reserve should probably be measured by flow rate [Figure 1].
In patients with a low SVi, a normal flow rate (≥ 200–211 ml/s) most likely indicated true SAS and a better outcome after valve intervention compared with those with a low flow rate in whom the true severity of AS remained uncertain. Conversely, in patients with low flow rate <200–211 ml/s and AVA <1 cm2, the threshold value of flow rate needs to be achieved to determine the true severity of AS. This is commonly facilitated by the use of DSE, which increases heart rate, resulting in shortening of the ejection time and an increase in flow rate even in the presence of a relatively unchanged SV.[6,21,22,23,24]
However, further studies are needed to validate the superiority of flow rate over SV in LF-LG-AS patients.
Projected aortic valve area a new index to improve identification of true-severe aortic stenosis
Several patients exhibit discordant findings on DSE (e.g. peak stress mean gradient of 29 mmHg and AVA of 0.8 cm2), thereby raising uncertainty about the actual severity of the stenosis. In this context, it is important to underline that all parameters of stenosis severity, including gradient and (to a lesser extent) AVA, are inherently flow dependent.[25] Hence, the changes in gradient and AVA during DSE largely depend on the magnitude of flow augmentation achieved by DSE, which may vary considerably from one patient to another.[19,25,26] The AVA and gradient are thus measured at flow conditions that differ dramatically from one patient to another and the utilization of these traditional parameters, which are not normalized to flow increase, may be misleading. To overcome this important limitation, a new parameter has been proposed: the projected AVA (AVAProj) at a normal transvalvular flow rate.[19,25,27] The purpose of this parameter is to estimate what would be the AVA at a standardized normal flow rate of 250 mL/s [Figure 2]. It has been found that in patients with LG-AS, the AVAProj better predicts underlying AS severity, impairment of myocardial blood flow, LV pump reserve, and survival compared to traditional DSE parameters.[19,25,27,28,29,30] The AVAProj has, thus, the potential to improve the diagnostic accuracy of DSE to distinguish SAS from MAS.
Figure 2.
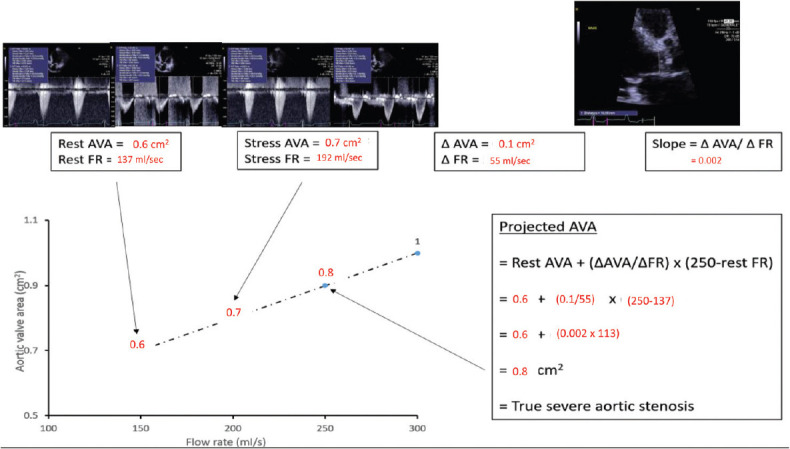
Projected aortic valve area calculation derived from resting and low-dose dobutamine echocardiography. Eighty-year-old woman with classical low-flow low-gradient severe aortic stenosis, ejection fraction of 29% and body surface area of 1.55 m2. Although stroke volume increased minimally with DSE, the flow rate increased 40% due to shortening of the ejection time, but MG and aortic valve area discordance persisted. In this example, the resting aortic valve area is 0.6 cm2 and the flow rate is 137 mL/s. The same measurements obtained during inotropic stress with low dose dobutamine give an aortic valve area of 0.7 cm2 and flow rate of 192 mL/s. Therefore, the flow rate has not normalized to at least 250 mL/s. The rate of increase in aortic valve area per unit change in flow rate is then derived from the two sets of data dividing the change in aortic valve area by the change in flow rate from rest to stress (slope of the line) = 0.002. Accordingly, the projected aortic valve area at the normalized flow rate equates to 0.8 cm2, indicating true severe aortic stenosis
When using the assessment of weight and calcification of the surgically explanted valves as the reference, the most discriminative cutoff value of AVAProj to identify SAS was <1.0 cm2[19,25,30] which is consistent with the traditional cutoff value proposed in the guidelines for SAS.[11,12] However, in the subset of patients with low EF and treated medically, a significant impact on survival was observed for AVAProj≤1.2 cm2.
These observations underscore the concept that, in fine, the outcome of these patients is primarily related to the imbalance between myocardial impairment and AS severity and that a MAS may thus be well tolerated by a ventricle with normal systolic function, but not by a failing ventricle where it may come to have the same impact as SAS in a normal ventricle. It is important to underline that the cutoff value of AVA <1.0 cm2 proposed in the guidelines for severe AS was initially established in a series of patients with preserved EF. In light of these observations, it might be more appropriate to use a higher cutoff value of AVAProj(e.g. ≤1.2) to recommend AVR in patients classical LG-AS. We will test this hypothesis in our study with a larger number of patients.
Aortic valve calcium score by multislice computed tomography, a flow-independent measure of stenosis severity
In patients with no LV pump reserve, who represent approximately 30%–40% of the patients with classical LF-LG-AS, there is no or very limited increase in transvalvular flow rate, and in this case, the stenosis severity often remains indeterminate at the outset of DSE.[13,26] More in patients with normal EF, DSE could be challenging in low-flow state patients and not useful in normal-flow patients. Quantification of valve calcification by MDCT [Table 1 and Figure 3] may be useful to distinguish SAS from MAS in these patients. Cueff et al. have suggested that a multislice computed tomography calcium score >1650 AU provides the best accuracy to identify SAS in patients with classical LF-LG-AS.[31] However, thresholds value for aortic valve calcification are sex specific: men >2000 AU and women >1200 AU, aortic valve calcium density (i.e., calcium score divided by aortic annulus area) ≥500 AU/cm2 in men, ≥300 AU/cm2 in women).[32]
Table 1.
Accuracy of aortic valve calcium score measured by multidetector computed tomography to predict severe aortic stenosis in patients with normal left ventricular outflow
| Aortic valve calcium score measured by CT (n=518) | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sensitivity (%) | Specificity (%) | AUC | Cutoff | Sensitivity (%) | Specificity (%) | |
| Valve calcium score | 0.93 | 2067 AU | 93 | 81 | 0.92 | 1172 AU | 88 | 87 |
| Valve calcium score/aortic annulus CSA | 0.95 | 510 AU/cm2 | 92 | 85 | 0.93 | 292 AU/cm2 | 92 | 83 |
Severe AS was defined as an AVA ≤1.0 cm2 and a mean gradient ≥40 mmHg. Normal LV outflow was defined as a stroke volume index >35 mL/m2 and a mean transvalvular flow rate >210 ml/s. These data were prospectively collected in 3 centers: QHLI, Bichat Hospital, and Mayo Clinic (31–34). ROC=Receiver operating characteristic, AUC=Area under the ROC curve, CSA=Cross-sectional area, SAVR=Surgical aortic valve replacement, LV=Left ventricular, AVA=Aortic valve area, QHLI=Quebec heart and lung institute
Figure 3.
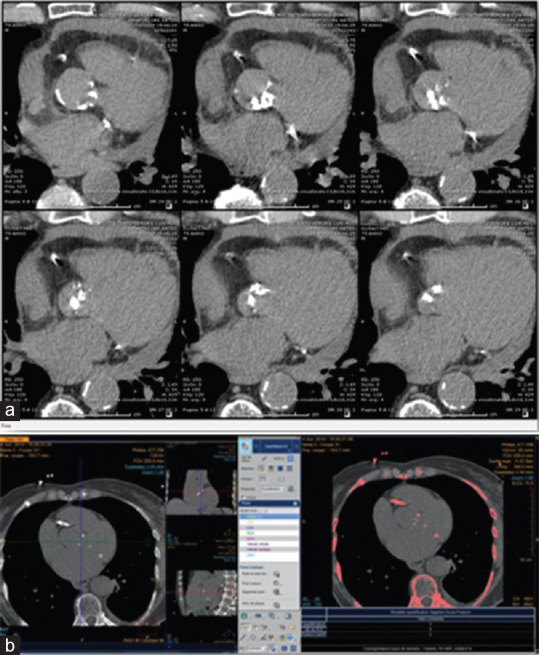
Measurement of aortic valve calcification by multi-detector computed tomography. Noncontrast multislice computed tomography showing axial view of the aortic valve, the axial multiplanar reformat images from left ventricular outflow tract to aortic direction (a) with any calcification highlighted in pink by the software (bone, coronary arteries, aorta, mitral annulus, (b). The region of the aortic valve is assessed in contiguous axial slices during held inspiration, at 120 kV tube voltage, pitch adjusted to heart rate (average 0.7), 64 mm × 0.6 mm collimation, and reconstruction slice thickness of 3 mm and increment of 1.5 mm. State-of-the-art dose reduction strategies including adjusting tube current to chest wall morphology, prospective electrocardiographic gating, and dose modulation should be used
The 2017 ESC guidelines also propose thresholds beyond which the stenosis is very likely severe (≥1600 AU in women and ≥3,000 AU in men) and thresholds below which the stenosis is very likely not severe (<800 AU in women and <1600 AU in men).[12]
The thresholds of valve calcification by MDCT have also been validated by outcome studies demonstrating that patients with severe calcific burden experience excess mortality under medical management.[33,34]
Indeed, in patients with severe calcium score, one can conclude that AS is severe. Since the first evidence of the diagnostic and prognostic value of MDCT calcium score are relatively recent, that its acquisition and analysis require a certain degree (although modest) of training and expertise, and its less-than-universal availability compared with echocardiography, this technique has not yet been invariably accepted. In particular, data on the utilization of MDCT valve calcium score to differentiate SAS versus MAS and/or predict outcome in patients with paradoxically LF-LG-AS and NF-LG-AS are scarce and need to be validated.
The valvuloarterial impedance, a new index to assess the global left ventricular hemodynamic load
The development of myocardial fibrosis and dysfunction and adverse events should logically be related to the global hemodynamic burden faced by the LV. To assess the global LV hemodynamic load that not only includes the valvular load but also the pulsatile and steady components of the arterial load, Hachicha et al. proposed a new Doppler-echo parameter: the valvuloarterial impedance (Zva= [SBP + MG]/SVi). In retrospective studies, Zva is a powerful predictor of survival in asymptomatic patients with AS.[35] We believe that the Zva may improve the risk stratification and clinical conduct in patients with LG-AS.
METHODS
Study design and sample size
The Discordant Echocardiographic Grading in Low-Gradient Aortic Stenosis (DEGAS Study) of the Italian Society of Echocardiography and Cardiovascular Imaging (SIECVI) is a prospective, multicenter, observational study that will enroll consecutive adult patients with LG-AS over 2 years. The follow-up period will last 12 months for the last included patient up to 36 months for the first included patient. Fifteen to twenty centers will be appointed and accepted voluntarily. It is expected that 15–20 consecutive patients with discordant echocardiographic findings and suspected SAS will be observed per center per year. Accordingly, ~ 300 patients should be enrolled for over 12 months. This sample size should allow us to generate hypotheses to improve our therapeutic approach to AS.
The IBM-Sample Power ver. 3.0 software will be used to calculate the sample size; sampling tests will be accepted at the power level β ≥80%, α = 5%, and tests with two tails.
Aims
The DEGAS registry aims to derive a data set of unselected patients with LG-AS, reaching the largest population ever reported on a national scale. The specific aims are:
To identify the occurrence of true SAS according to EF/flow pattern
To validate the use of aortic valve calcification as measured by MDCT
To assess the interest of flow rate, global longitudinal strain, and diastolic dysfunction in LG-AS patients
To assess the usefulness of NT pro-BNP and high-sensitive troponin
To assess the interest of valvuloarterial impedance in LG-AS patients.
Endpoints
Prevalence of true AS severity: AS severity will be confirmed by a multimodality approach including DSE and aortic calcium score at MDCT according to investigators' experience. However, one of them (MDCT) will be enough for including a patient in case it performs convincing results
Clinical outcomes: the primary clinical outcome variable will be 12-month all-cause mortality as recommended by the VARC.[36] The secondary outcome variables will be (i) 30-day mortality (for patients treated by Surgical AVR or TAVR); (ii) 12-month cardiovascular mortality; (iii) 12-month new major cardiovascular events as defined by VARC (myocardial infarction, stroke, vascular complications, and rehospitalization for heart failure); and (iv) composite endpoint of cardiovascular mortality and hospitalization for heart failure.
Primary study hypotheses
Fifty percent or more of patients with LG-AS have a SAS. This proportion will be higher in low EF patients
Aortic valve calcification will be predictive of events in patients with LG-AS.
Secondary study hypotheses
Global longitudinal strain and diastolic dysfunction parameters will be predictive of events in LG-AS patients
The AVAProj measured by DSE will be superior to the conventional indices of stenosis severity (rest or peak stress AVA and gradient) for the discrimination of true severe versus pseudo severe AS (determined by calcification) and the prediction of hemodynamic/functional/clinical outcomes in LF-LG-AS patients
The valvuloarterial impedance will be useful to predict an adverse event in LF-LG-AS patients and will correlate with NT pro-BNP
LV pump reserve (stress-induced increase in SV) will not be able to predict operative/procedural risk and hemodynamic/functional/clinical outcomes in LF-LG-AS opposed when measured by an increase in flow rate.
Inclusion criteria
(1) Age >21 years; (2) suspected SAS defined by an AVA ≤1.0 cm2 and indexed AVA ≤0.6 cm2/m2; and (3) low transvalvular gradient defined by a mean gradient <40 mmHg.
Exclusion criteria
(1) > Mild aortic regurgitation, > mild mitral stenosis, > moderate mitral regurgitation; (2) end-stage renal disease; (3) pregnant or lactating women; and (4) unwillingness to provide informed consent.
Systemic hypertension is frequent in patients with LG-AS and may contribute to the reduced flow (and thus to the low gradient). If the patient is hypertensive, antihypertensive therapy should be instituted or optimized and the clinical and echocardiographic data should be reassessed after normalization of blood pressure.[35]
Patients diagnosed with transthyretin amyloidosis can be included. Patients having a coronary artery disease that is requiring revascularization at the time of baseline echocardiography can be included. However, for all the patients, it is mandatory to identify possible causes of low-flow (e.g., atrial fibrillation) and reassess parameters of stenosis before to proceed with inclusion.
Current or previous participation in cardiovascular or non-cardiovascular trials is not excluding the patient from participation in the DEGAS study.
Baseline studies
Medical history, physical examination, and functional capacity
Medical history, concomitant risk factors and diseases, current medication, weight, height, blood pressure, symptoms, and functional status (NYHA class) will be determined.[37] A 6-min walk test (6MWT) will also be performed to provide a more objective assessment of the patient's functional capacity.[38]
Biomarkers (optional)
Plasma levels of NT pro-BNP (NT proBNP) and high-sensitive troponin will be measured using established radioimmunoassay.
Doppler-echocardiography
The echocardiographers at each site will use the standardized acquisition of the echocardiograms. Left ventricular systolic function will be assessed by biplane Simpson's EF. Left ventricular pump function: SV will be measured in the left ventricular outflow tract (LVOT) (at the aortic anulus, using the midsystolic image that bisects the largest dimension of the aortic annulus (i.e., the plane that bisects the right coronary cusp point hinge point anteriorly and the interleaflet triangle between the left and noncoronary cusps posteriorly,[39] mean transvalvular flow rate (Q) will be calculated by dividing SV by LV ejection time. LV diastolic function will be assessed as previously described.[40] Aortic valve function: peak aortic jet velocity, AVA by continuity equation, peak and mean transvalvular gradients by Bernoulli formula. Postextrasystolic potentiation-associated augmentation in peak and mean transvalvular gradients should be evaluated in case of incidental premature ventricular contraction during resting echocardiography.[41,42] The global LV hemodynamic load resulting from the valvular and arterial loads will be assessed using the valvuloarterial impedance.[35,42] Global longitudinal strain (optional) will be assessed as previously described.[43] 3D echo (optional): 3D EF and 3D SV will be recorded.
Dobutamine stress echocardiography (optional)
Classical LG-AS patients will undergo a DSE to assess (1) LV pump reserve (i. e., stress-induced increase in SV and mean flow rate) and (2) stenosis severity. The dobutamine infusion protocol consists of 5 min increments of 5 μg/kg/min up to a maximum dosage of 20 μg/kg/min and echo measurements are performed at each stage.[25] The endpoints for terminating DSE are (1) heart rate >220-age; (2) systolic blood pressure <80 or >220 mmHg; (3) significant increase in the LVOT gradient; (4) ischemia detected by electrocardiographic (>5 mm of flat or downsloping ST depression);[44] (5) complex ventricular arrhythmias or rapid new atrial arrhythmias; (6) breathlessness, angina, dizziness, or syncope, and (7) maximum dose reached (20 μg/kg/min). After each increment in dobutamine dose, a period of 5 min is allowed to ensure the stabilization of hemodynamic status before starting the measurements that include SV, mean flow rate, AVA, gradients, systolic/diastolic blood pressures, and Zva. The AVAproj is determined as described.[25]
Multidetector computed tomography
Image analysis will be performed locally using a range of different software packages. At the initiation of the study, the consensus will be achieved on the optimum method for calcium scoring, and this will be then applied at each of the centers, ensuring consistency of approach. The typical radiation dose associated with this study will be 0.8–1.0 mSv, less than the yearly radiation exposure from natural sources as reported by the Princeton group.[45] Off-line image analysis will be conducted on dedicated workstations using validated software by modified Agatston.[32] Total valve calcium score will be calculated by summing the per-slice lesion scores for all sections containing calcium and excluding coronary and non-valvular calcifications [Figure 3]. The calcium score will be indexed to the aortic annulus cross-sectional area measured by MDCT to assess the “calcification density.”[46]
Therapeutic decision, management, and follow-up
Decisions on drug prescriptions and indications to perform diagnostic and therapeutic procedures will be left to participating cardiologists who will know the baseline measures of traditional parameters of disease severity, according to international guidelines and good clinical practice. No specific protocols or recommendations for treatment will be made during this observational study. Therefore, there will be no attempt to interfere with the routine clinical care of the patient who, according to the disease's condition, will be expected to attend at least one visit during the follow-up. A visit close to 12 ± 3 months after the in- or outpatient entry visit will be recommended to collect information on morbidity and mortality. A phone call can replace the follow-up clinical visit in cases where the patient cannot attend the center for clinical or logistical reasons.
Statistical analyses
Data will be expressed as mean ± standard deviation, median/range, or as proportions. Categorical data will be analyzed using Chi-Square or Fisher's exact test. Correlations between variables will be expressed using the Pearson's or Spearman's correlation coefficients. Continuous variables will be analyzed using a t-Student or ANOVA followed by Tukey's test. The normality assumption will be verified using the Shapiro–Wilk test. Data will be investigated for log-transformed to satisfy this assumption. Multivariable linear (continuous variables) or logistic (dichotomous variables: e.g., MAS vs. SAS; presence vs. absence of LV pump reserve; global longitudinal strain) regression analyses and multivariable Cox regression analysis will be used. Variables previously reported as being associated with the studied endpoints and those with a P < 0.1 on univariate analysis will be entered into the models. We will also analyze the interactions between the following variables to their impact on outcomes: age, sex, stenosis severity (SAS vs. MAS), degree of myocardial impairment (i.e., LV pump or contractile reserve; global longitudinal strain), and type of treatment.
Ethical issues
All centers will require local ethics approval. All patients will be approached by the center investigator and will be asked for their written informed consent to participate in the DEGAS study on AS. No data will be collected before written detailed information is given to the patient and signed informed consent is obtained.
In centers where written informed consent is not mandatory for patient participation in a registry, written informed consent will not be required but this should be documented in the ethics application and approved by the ethics board, according to the local rules.
For those patients who will be admitted with the severe clinical conditions and not able to consent at the time of admission, information and written consent will be obtained from a legally authorized representative if allowed by the ethics board. Patients will have to give consent as soon as more favorable clinical conditions allow them to receive appropriately the study information. The patient or legally acceptable representative will be given a copy of the signed informed consent.
Protection of human subjects
The DEGAS registry will not require the transmission of identification data outside the participating centers. The data collected will be anonymous. Each patient will be assigned a unique identification number and no other identification variables will be entered. The identity of the patient will remain at the participating center as confidential information. Information aimed at identifying the individual patients of the study will not be collected or stored in the database. All confidential information will be password protected for electronic data or stored in secure places for paper data. For these reasons, a high level of security will be assured. To maintain these high levels of security at the same time as data reliability, each researcher will have a single personal login and password to access patient information. There will not be a collection of data outside the collection tools, which will take place through a web platform (REDCap), absolutely secure based on current standards concerning the ethical requirements and data integrity.
Pharmacovigilance
In this observational study, there are no diagnostic or therapeutic interventions other than those already recommended by contemporary guidelines [Figure 4].
Figure 4.
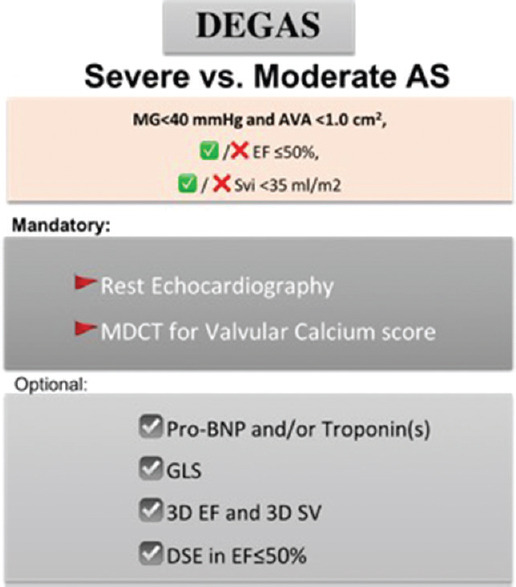
Summary scheme of the mandatory and optional exams of the Discordant Echocardiographic Grading in Low-Gradient Aortic Stenosis study
Quality control of enrolling centers
As with any imaging modality, the quantification of valve calcification by MDCT is not a perfect method to assess AS severity.[47]
However, the use of different scanners for image acquisition and different software for image analysis is reported not to have any significant effect on thresholds levels or accuracy of valve calcification (Area under the curve: ≥0.89) to identify severe AS.[32,33]
Nevertheless, quality control of MDCT diagnostic performance among the enrolling centers will be of critical importance to acquire meaningful information into the data bank and to reduce interobserver variability. For this purpose, a video tutorial prepared by the steering committee will be available on https://www.siec.it/ricerca which elucidates questions concerning the quantification of valve calcification by MDCT and the methods of measurement. The second criterion will consist in random sampling of 5 consecutive studies from each contributing center. These 5 studies will be examined in a blinded fashion by two members of the steering committee who will verify the adequacy and congruence of the data entered in the database.
Timeline
An invitation letter will be sent to all members of the SIECVI. In case of preliminary interest, you must notify us by E-mail (ricerca@siecvi.it) by 31 August 2020 providing the following information: institute name, name of the local PI, E-mail and telephone contact. After the promoting center and local ethics approval we will start recruiting patients.
DISCUSSION
The general objective of the DEGAS study will be to validate new parameters to improve the assessment of AS severity and the clinical decision-making in patients with LG-AS presenting to cardiology centers in Italy who will be interested in taking part in the study. It will involve cardiology units that regularly follow and/or admit patients with AS.
In patients with paradoxical LF-LG-AS, the LV pump is considered normal. Therefore, EF is unable to identify patients with poorer outcomes. More sensitive markers of systolic dysfunction such as global longitudinal strain are needed to predict a worse outcome.[43,48] Moreover, those patients often suffer from diastolic dysfunction and E, A. e' wave could be of importance to stratify them.[49]
Nevertheless, it should be underlined that several uncertainties persist about the optimal treatment of LG-AS in clinical practice. A recent survey evidenced that only half of the centers reported the routine calculation of the SVi in these patients in Europe, indicating limited adherence to ESC guidelines.[50]
Despite these perplexities, no prospective randomized trial has still conducted to evaluate the optimal management of patients with LG-AS. Of note, there are also some contemporary data suggesting that moderate AS (i.e., pseudo-severe AS) may not be a benign stage in the evolution of the disease as the conventional wisdom tells us almost in patients at an advanced stage of cardiac damage and high risk of rapid progression of AS.[51]
Therefore, it becomes increasingly emerging to collect data from prospective registers.
True- or pseudo-severe aortic stenosis crucial information for clinical decision-making
Mean gradient and peak velocity require careful and precise attention as errors in measurement may lead to the erroneous conclusion of discordant grading. In this perspective, the right parasternal window interrogation is crucial to properly assess the AS severity. Recently, Benfari et al. showed that the right parasternal view is feasible in 83% of cases.[52] Of note, when evaluated from the right parasternal view versus apical view, MG is higher in 80% of cases resulting in a reclassification of severity toward moderate to severe AS in 15% of patients according to MG criteria.[53]
The evaluation of the changes in AVA and gradient during DSE are helping differentiate SAS from MAS.[13,25,26,54,55] Typically, AVA increases to a larger extent with the increasing flow in MAS because the valve is less rigid than in SAS whereby there is little or no increase in AVA and a marked increase in gradient in response to increasing flow [Figure 1]. The prevalence of MAS reported in previous studies is comprised between 20 and 30% in classical LF-LG-AS and 30-50% in paradoxical LG-AS.[19,25,26,32,56,57] Several DSE parameters and criteria have been proposed in the literature to identify patients with MAS with low EF including: peak stress mean gradient ≤30 or <40 mmHg depending on the studies, peak stress AVA >1.0 or 1.2 cm2, and/or an absolute increase in AVA ≥0.3 cm2.[14,26,54,56,58] Among these criteria, the one that would appear to have the best predictive value to identify MAS is a combination of a peak stress AVA ≥1.2 cm2 and a peak stress gradient <40 mmHg [Figure 1].[19,25,56] The accuracy, and in particular, the sensitivity of these criteria, however, remains suboptimal.[19,25,27] None have been proposed to identify MAS in patients with paradoxically LG-AS. For all these reasons, the flow rate could be a better marker than SV and the quantification of valve calcification by MDCT represents a faster and potentially easier alternative that is both feasible and conclusive in the vast majority of patients.
CONCLUSION
A multimodality imaging approach should be considered to confirm AS severity and indication of intervention in any symptomatic patient with discordant grading at rest echocardiography, regardless of the EF and flow status. However, there have been very few prospective studies performed until now in patients with LG-AS and these studies have included a relatively small number (<100) of patients and have often used only one imaging modality (basal echocardiography). Our prospective study is the first of its kind, as it will use a complementary multimodality imaging approach to measure traditional parameters of disease severity. This study shall contribute to improve the diagnostic evaluation and clinical conduct in patients with LG-AS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dulgheru R, Pibarot P, Sengupta PP, Piérard LA, Rosenhek R, Magne J, et al. Multimodality imaging strategies for the assessment of aortic stenosis: Viewpoint of the heart valve clinic international database (HAVEC) Group. Circ Cardiovasc Imaging. 2016;9:e004352. doi: 10.1161/CIRCIMAGING.115.004352. [DOI] [PubMed] [Google Scholar]
- 2.Everett RJ, Clavel MA, Pibarot P, Dweck MR. Timing of intervention in aortic stenosis: A review of current and future strategies. Heart. 2018;104:2067–76. doi: 10.1136/heartjnl-2017-312304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delgado V, Clavel MA, Hahn RT, Gillam L, Bax J, Sengupta PP, et al. How do we reconcile echocardiography, computed tomography, and hybrid imaging in assessing discordant grading of aortic stenosis severity? JACC Cardiovasc Imaging. 2019;12:267–82. doi: 10.1016/j.jcmg.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Ternacle J, Clavel MA. Assessment of Aortic Stenosis Severity: A Multimodality Approach. Cardiol Clin. 2020;38:13–22. doi: 10.1016/j.ccl.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Guzzetti E, Pibarot P, Clavel MA. Normal-flow low-gradient severe aortic stenosis is a frequent and real entity. Eur Heart J Cardiovasc Imaging. 2019;20:1102–4. doi: 10.1093/ehjci/jez211. [DOI] [PubMed] [Google Scholar]
- 6.Saeed S, Vamvakidou A, Seifert R, Khattar R, Li W, Senior R. The impact of aortic valve replacement on survival in patients with normal flow low gradient severe aortic stenosis: A propensity-matched comparison. Eur Heart J Cardiovasc Imaging. 2019;20:1094–101. doi: 10.1093/ehjci/jez191. [DOI] [PubMed] [Google Scholar]
- 7.Thaden JJ, Balakrishnan M, Sanchez J, Adigun R, Nkomo VT, Eleid M, et al. Left ventricular filling pressure and survival following aortic valve replacement for severe aortic stenosis. Heart. 2020;106:830–7. doi: 10.1136/heartjnl-2019-315908. [DOI] [PubMed] [Google Scholar]
- 8.Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, et al. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J Am Coll Cardiol. 2014;63:2016–25. doi: 10.1016/j.jacc.2014.02.581. [DOI] [PubMed] [Google Scholar]
- 9.Dahou A, Clavel MA, Capoulade R, O'Connor K, Ribeiro HB, Côté N, et al. B-Type natriuretic peptide and high-sensitivity cardiac troponin for risk stratification in low-flow, low-gradient aortic stenosis. Jacc Cardovasc Imaging. 2018;11:939–47. doi: 10.1016/j.jcmg.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, et al. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: Relationship to hemodynamics and clinical outcome: Results from the Multicenter Truly or Pseudo-Severe Aortic Stenosis (TOPAS) study. Circulation. 2007;115:2848–55. doi: 10.1161/CIRCULATIONAHA.106.654210. [DOI] [PubMed] [Google Scholar]
- 11.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease).Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1–142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 13.Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardio. 2009;54:2251–60. doi: 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 14.Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, et al. Low-gradient aortic stenosis: Operative risk stratification and predictors for long-term outcome: A multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–24. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- 15.Quere JP, Monin JL, Levy F, Petit H, Baleynaud S, Chauvel C, et al. Influence of preoperative left ventricular contractile reserve on postoperative ejection fraction in low-gradient aortic stenosis. Circulation. 2006;113:1738–44. doi: 10.1161/CIRCULATIONAHA.105.568824. [DOI] [PubMed] [Google Scholar]
- 16.Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, et al. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: A European multicenter study. J Am Coll Cardiol. 2008;51:1466–72. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 17.Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–73. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: The TOPAS-TAVI registry. J Am Coll Cardiol. 2018;71:1297–308. doi: 10.1016/j.jacc.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 19.Clavel MA, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, et al. Validation of conventional and simplified methods to calculate projected valve area at normal flow rate in patients with low flow, low gradient aortic stenosis: The multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) study. J Am Soc Echocardiogr. 2010;23:380–6. doi: 10.1016/j.echo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Senior R, Khattar RS. Assessment of aortic stenosis: Time to go with the flow. J Am Coll Cardiol. 2020;75:1770–1. doi: 10.1016/j.jacc.2020.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Namasivayam M, He W, Churchill TW, Capoulade R, Liu S, Lee H, et al. Transvalvular flow rate determines prognostic value of aortic valve area in aortic stenosis. J Am Coll Cardiol. 2020;75:1758–69. doi: 10.1016/j.jacc.2020.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chahal NS, Drakopoulou M, Gonzalez-Gonzalez AM, Manivarmane R, Khattar R, Senior R. Resting aortic valve area at normal transaortic flow rate reflects true valve area in suspected low-gradient severe aortic stenosis. JACC Cardiovasc Imaging. 2015;8:1133–9. doi: 10.1016/j.jcmg.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Vamvakidou A, Jin W, Danylenko O, Chahal N, Khattar R, Senior R. Low transvalvular flow rate predicts mortality in patients with low-gradient aortic stenosis following aortic valve intervention. JACC Cardiovasc Imaging. 2019;12:1715–24. doi: 10.1016/j.jcmg.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Vamvakidou A, Jin W, Danylenko O, Pradhan J, Li W, West C, et al. Impact of pre-intervention transaortic flow rate versus stroke volume index on mortality across the hemodynamic spectrum of severe aortic stenosis: Implications for a new hemodynamic classification of aortic stenosis. JACC Cardiovasc Imaging. 2019;12:205–6. doi: 10.1016/j.jcmg.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: The multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation. 2006;113:711–21. doi: 10.1161/CIRCULATIONAHA.105.557678. [DOI] [PubMed] [Google Scholar]
- 26.deFilippi CR, Willett DL, Brickner ME, Appleton CP, Yancy CW, Eichhorn EJ, et al. Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol. 1995;75:191–4. doi: 10.1016/s0002-9149(00)80078-8. [DOI] [PubMed] [Google Scholar]
- 27.Annabi MS, Touboul E, Dahou A, Burwash IG, Bergler-Klein J, Enriquez-Sarano M, et al. Dobutamine stress echocardiography for management of low-flow, low-gradient aortic stenosis. J Am Coll Cardiol. 2018;71:475–85. doi: 10.1016/j.jacc.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 28.Burwash IG, Lortie M, Pibarot P, de Kemp RA, Graf S, Mundigler G, et al. Myocardial blood flow in patients with low-flow, low-gradient aortic stenosis: Differences between true and pseudo-severe aortic stenosis.Results from the multicentre TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Heart. 2008;94:1627–33. doi: 10.1136/hrt.2007.135475. [DOI] [PubMed] [Google Scholar]
- 29.Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, et al. Predictors of outcomes in low-flow, low-gradient aortic stenosis: Results of the multicenter TOPAS Study. Circulation. 2008;118:S234–42. doi: 10.1161/CIRCULATIONAHA.107.757427. [DOI] [PubMed] [Google Scholar]
- 30.Clavel MA, Ennezat PV, Maréchaux S, Dumesnil JG, Capoulade R, Hachicha Z, et al. Stress echocardiography to assess stenosis severity and predict outcome in patients with paradoxical low-flow, low-gradient aortic stenosis and preserved LVEF. JACC Cardiovasc Imaging. 2013;6:175–83. doi: 10.1016/j.jcmg.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, et al. Measurement of aortic valve calcification using multislice computed tomography: Correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–6. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 32.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, et al. The complex nature of discordant severe calcified aortic valve disease grading: New insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol. 2013;62:2329–38. doi: 10.1016/j.jacc.2013.08.1621. [DOI] [PubMed] [Google Scholar]
- 33.Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging. 2018;11:e007146. doi: 10.1161/CIRCIMAGING.117.007146. [DOI] [PubMed] [Google Scholar]
- 34.Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: Results of an international registry study. J Am Coll Cardiol. 2014;64:1202–13. doi: 10.1016/j.jacc.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–11. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 36.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: A consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 38.Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed) 1986;292:653–5. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn RT, Pibarot P. Accurate measurement of left ventricular outflow tract diameter: Comment on the updated recommendations for the echocardiographic assessment of aortic valve stenosis. J Am Soc Echocardiogr. 2017;30:1038–41. doi: 10.1016/j.echo.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Dumesnil JG, Paulin C, Pibarot P, Arsenault M, Coulombe D. Mitral annulus velocities by tissue Doppler imaging: Practical implications with regards to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15(10 Pt 2):1226–31. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 41.Garcia D, Pibarot P, Dumesnil JG, Sakr F, Durand LG. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation. 2000;101:765–71. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 42.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 43.Dahou A, Bartko PE, Capoulade R, Clavel MA, Mundigler G, Grondin SL, et al. Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low-gradient aortic stenosis: Results from the multicenter True or Pseudo-Severe Aortic Stenosis study. Circ Cardiovasc Imaging. 2015;8:e002117. doi: 10.1161/CIRCIMAGING.114.002117. [DOI] [PubMed] [Google Scholar]
- 44.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, et al. Prospective study of asymptomatic valvular aortic stenosis.Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–70. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 45.University P Open Source Radiation Safety Training Module 2: Background Radiation & Other Sources of Exposure; 2011. [[Last accessed on 2020 Jun 20]]. Available from: http://webprincetonedu/sites/ehs/osradtraining/backgroundradiation/backgroundhtm#nbr .
- 46.Pawade T, Sheth T, Guzzetti E, Dweck MR, Clavel MA. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019;12:1835–48. doi: 10.1016/j.jcmg.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 47.Shen M, Tastet L, Capoulade R, Larose É, Bédard É, Arsenault M, et al. Effect of age and aortic valve anatomy on calcification and haemodynamic severity of aortic stenosis. Heart. 2017;103:32–9. doi: 10.1136/heartjnl-2016-309665. [DOI] [PubMed] [Google Scholar]
- 48.Lancellotti P, Donal E, Magne J, Moonen M, O'Connor K, Daubert JC, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: The importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–71. doi: 10.1136/hrt.2009.190942. [DOI] [PubMed] [Google Scholar]
- 49.Dahou A, Clavel MA, Bartko P, Capoulade R, Mundigler G, Bergler-Klein J, et al. Moderate/severe diastolic dysfuction is associated with worse outcome in patients with low-flow, low-gradient aortic stenosis and low-ejection fraction-Results of the TOPAS study. Can J Cardiol. 2014;30:S243–4. [Google Scholar]
- 50.Michalski B, Dweck MR, Marsan NA, Cameli M, D'Andrea A, Carvalho RF, et al. The evaluation of aortic stenosis, how the new guidelines are implemented across Europe: A survey by EACVI. Eur Heart J Cardiovasc Imaging. 2020;21:357–62. doi: 10.1093/ehjci/jeaa009. [DOI] [PubMed] [Google Scholar]
- 51.Vannan MA, Pibarot P, Lancellotti P. Aortic stenosis: The emperor's new clothes. J Am Coll Cardiol. 2019;74:1864–7. doi: 10.1016/j.jacc.2019.08.1029. [DOI] [PubMed] [Google Scholar]
- 52.Benfari G, Mantovani F, Romero-Brufau S, Setti M, Rossi A, Ribichini FL, et al. The right parasternal window: When Doppler-beam alignment may be life-saving in patients with aortic valve stenosis? J Cardiovasc Med (Hagerstown) 2020 doi: 10.2459/JCM.0000000000000971. doi: 102459/JCM0000000000000971 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Benfari G, Gori AM, Rossi A, Papesso B, Vassanelli C, Zito GB, et al. Feasibility and relevance of right parasternal view for assessing severity and rate of progression of aortic valve stenosis in primary care. Int J Cardiol. 2017;240:446–51. doi: 10.1016/j.ijcard.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 54.Schwammenthal E, Vered Z, Moshkowitz Y, Rabinowitz B, Ziskind Z, Smolinski AK, et al. Dobutamine echocardiography in patients with aortic stenosis and left ventricular dysfunction: Predicting outcome as a function of management strategy. Chest. 2001;119:1766–77. doi: 10.1378/chest.119.6.1766. [DOI] [PubMed] [Google Scholar]
- 55.Monin JL, Monchi M, Gest V, Duval-Moulin AM, Dubois-Range JL, Gueret P. Aortic stenosis with severe left ventricular dysfunction and low transvalvular pressure gradients. J Am Coll Cardiol. 2001;37:2101–7. doi: 10.1016/s0735-1097(01)01339-0. [DOI] [PubMed] [Google Scholar]
- 56.Nishimura RA, Grantham JA, Connolly HM, Schaff HV, Higano ST, Holmes DR, Jr , Low-output, low-gradient aortic stenosis in patients with depressed left ventricular systolic function: The clinical utility of the dobutamine challenge in the catheterization laboratory. Circulation. 2002;106:809–13. doi: 10.1161/01.cir.0000025611.21140.34. [DOI] [PubMed] [Google Scholar]
- 57.Clavel MA, Côté N, Mathieu P, Dumesnil JG, Audet A, Pépin A, et al. Paradoxical low-flow, low-gradient aortic stenosis despite preserved left ventricular ejection fraction: New insights from weights of operatively excised aortic valves. Eur Heart J. 2014;35:2655–62. doi: 10.1093/eurheartj/ehu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuppiroli A, Mori F, Olivotto I, Castelli G, Favilli S, Dolara A. Therapeutic implications of contractile reserve elicited by dobutamine echocardiography in symptomatic, low-gradient aortic stenosis. Ital Heart J. 2003;4:264–70. [PubMed] [Google Scholar]