FIGURE 2.
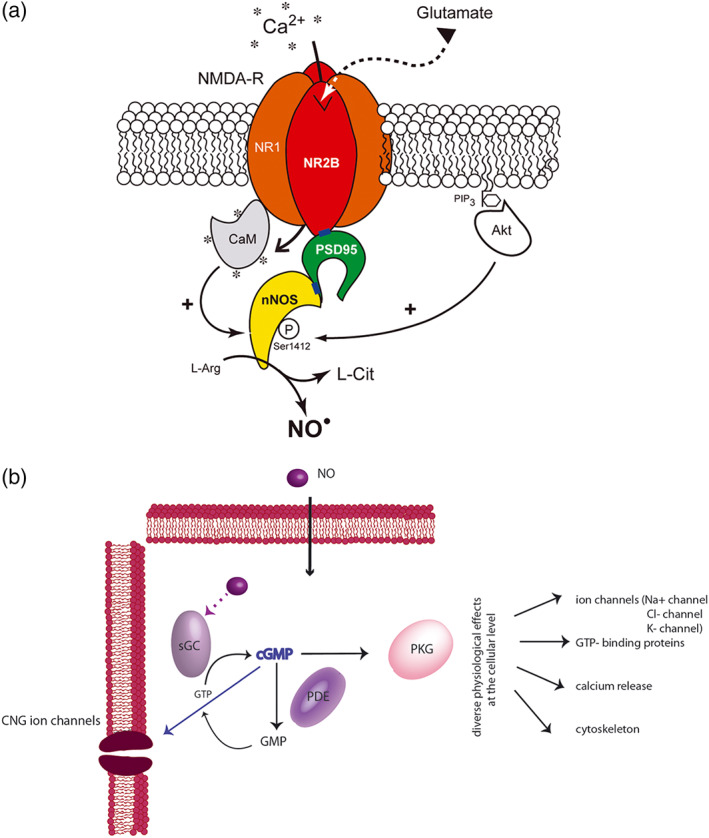
Schematic representation of the NO/cGMP signalling pathway. (a) The translocation of nNOS from the cytosol to the membrane, its physical interaction with the NR2B subunit of the NMDA receptor (NMDA‐R) via PDZ domains (blue rectangles) involves the postsynaptic density 95 (PSD‐95) scaffolding protein and the assembly of a ternary complex nNOS/PSD‐95/NMDA receptor. Binding of glutamate to the NMDA receptor allows Ca+2 entry into the neuron. Ca+2 influx activates nNOS α through calmodulin (CaM) binding leading to the production of NO, which is formed enzymically from L‐arginine (L‐Arg) in equimolar amounts with L‐citrulline (L‐Cit). In parallel, membrane‐tethered nNOS is also subjected to posttranscriptional modifications (such as phosphorylation via Akt) that modulates its catalytic activity (Adak et al., 2001; Guerra et al., 2019; Rameau et al., 2007). (b) NO is a highly soluble and membrane permeable neurotransmitter. Upon binding to NO‐sensitive guanylate cyclase, NO induces a conformational change resulting in the activation of the enzyme and the subsequent conversion of GTP to cGMP. The newly produced cGMP can interact with various intracellular proteins, including cGMP‐binding PDE, cGMP‐gated cation channels (CNGs), and PKG, triggering the phosphorylation of many different substrates. The NO/cGMP pathway is thus implicated in multiple distinct physiological processes such as cytoskeletal organization, Ca2+ release from intracellular stores, and differentiation/proliferation of vascular smooth muscle
