FIGURE 5.
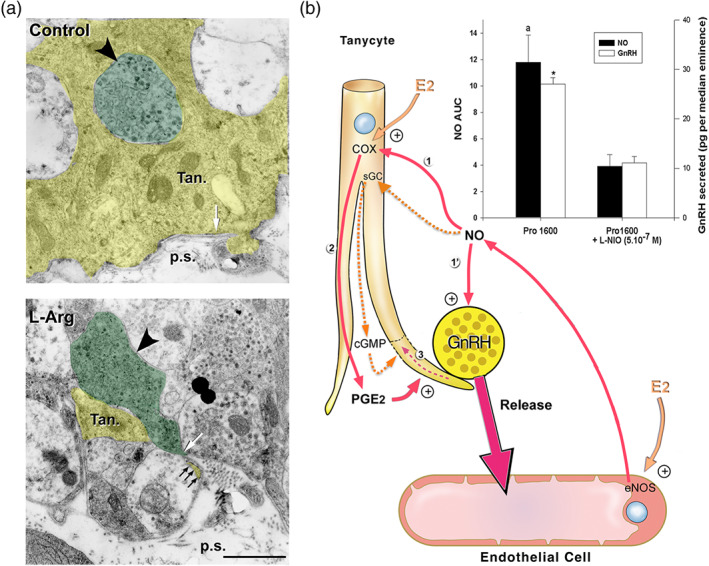
Involvement of eNOS and endothelial NO signalling in oestrous‐cycle‐mediated brain plasticity and neurosecretion in the neuroendocrine hypothalamus. (a) Activation of endogenous NO secretion in endothelial cells of the median eminence promotes tanycytic process retraction (yellow, Tan) that allows GnRH nerve terminals (green, big arrowhead) to form direct neurovascular junctions in isolated median eminence explants. Living median eminence explants were incubated for 30 min in the presence or absence of the NO precursor, L‐arginine (L‐Arg, 500 mM). Under basal unstimulated conditions (control), the GnRH axonal endings were separated from the pericapillary space (p.s.; delineated by the parenchymatous basal lamina, white arrow). Most of the nerve endings were enwrapped by a single tanycytic end‐foot (top panel, Tan). Retraction of tanycytic processes (white arrowhead) and formation of neurovascular junctions by GnRH nerve terminals that directly contact the pericapillary space (bottom panel, white arrow) were detected upon treatment with L‐Arg. Scale bar, 1 μm. From De Seranno et al. (2004) with permission. (b) Schematic representation of endothelial–glial–neuronal interactions involved in the control of GnRH neurohormone secretion in the hypothalamus. Endothelial–neuronal interactions at the level of the median eminence (the termination field of GnRH neurons in the hypothalamus) involves the production of NO by endothelial cells of fenestrated capillaries of the portal blood vessels. Upon its secretion, NO diffuses from its source, where it not only stimulates the release of GnRH from the neighbouring GnRH neuroendocrine terminals (1′; Knauf et al., 2001) but also promotes their access to the blood stream by inducing cytoarchitectural changes in tanycytic end‐feet (1–3; De Seranno et al., 2004). Importantly, on the afternoon of proestrus (when oestrogen levels are at their highest), the preovulatory GnRH/NO release is blocked with L‐N 5‐(1‐iminoethyl)ornithine (L‐NIO), an NOS inhibitor selective for eNOS at 0.5 μM (bar graph; * and a, significantly different from treated samples, P < .05: AUC; NO levels have been measured by amperometry during a 30‐min period in living median eminence explants; Knauf et al., 2001). Downstream effectors of endothelial NO‐meditated plasticity in tanycytes were shown to be both soluble GC (sGC) and COX (1). Note: Whether NO activates COX activity directly or indirectly is unknown (Garthwaite & Boulton, 1995); NO from endogenous sources stimulates PGE2 production via COX in a cGMP‐independent manner (Salvemini et al., 1995), alternatively NO could somehow prevent the auto‐inactivation of COX (Smith, Marnett, & DeWitt, 1991). Oestrogens are likely to be the key humoral factors involved in the orchestration of the endothelia‐to‐glia communication that allows GnRH neurons to directly contact the pituitary portal blood vessels on the day of proestrus (De Seranno et al., 2010). Oestrogens treatment up‐regulates COX expression while leaving unchanged the expression of sGC. In addition, COX products and PGE2 in particular (2) induce acute remodelling of actin cytoskeleton in tanycytes and cause cytoplasm retraction within tanycytic processes and end‐feet (3). In parallel, oestrogens stimulate endothelial NOS (eNOS) expression in median eminence endothelial cells. Both tanycytes and endothelial cells express oestrogen receptors in vitro (De Seranno et al., 2010) and in vivo (Giacobini et al., 2014; Parkash et al., 2015). Adapted from Prevot (2002) and Prevot et al. (2010) with permission
