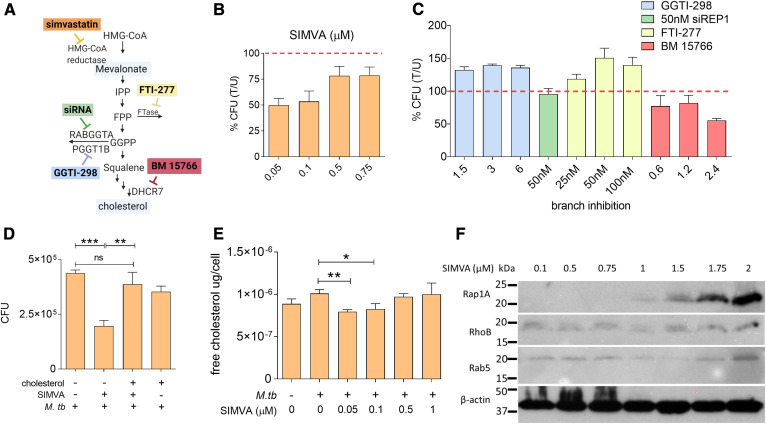
Fig. 1.
Simvastatin reduces the intracellular burden of M. tuberculosis by inhibiting cellular cholesterol biosynthesis. A: Diagram of the mevalonate pathway. IPP, isopentenyl pyrophosphate; FPP, farnesyl pyrophosphate; FTase, farnesyltransferase; GGPP, geranylgeranyl pyrophosphate; RABGGTA, Rab geranylgeranyltransferase subunit alpha; PGGT1B, protein geranylgeranyltransferase type I subunit beta; DHCR7, 7-dehydrocholesterol reductase. The colored boxes represent pharmacological inhibitors of specific enzymes in the pathway (simvastatin, FTI-277, GGTI-298, BM 15766), as indicated. siRNA targeting REP-1 (a chaperone protein that presents Rab proteins for prenylation by GGTase-II) was used because no pharmacological inhibitor of GGTI type 2 was commercially available. B, C: THP1 cells were infected with M. tuberculosis H37Rv, followed by treatment with multiple doses of simvastatin (B) or branch inhibitors of the mevalonate pathway (C). CFUs were enumerated at 6 days post treatment. Data are presented as percent CFU changes relative to solvent control. U, solvent-treated; T, treated. The red dashed line represents M. tuberculosis CFU levels in the absence of any pharmacological treatment. D: Analysis of cellular cholesterol levels in infected THP1 cells treated with solvent and increasing doses of simvastatin. (E) Intracellular growth of M. tuberculosis in THP1 cells treated for 6 days with 100 nM simvastatin in the absence and presence of water-soluble cholesterol (1.25 μg/ml). Significance was tested by Student’s t-test: *P < 0.05; **P < 0.01 and ***P < 0.001. F: Western blot analysis of sentinel protein targets of the mevalonate pathway of infected THP1 cells treated with simvastatin (SIMVA; 0.1–2 μM) for 6 days. β-Actin was used as loading control. It is noted that, in particular, the antibody against Rap1A is directed against the unprenylated Rap1A form, and therefore, the signal increases with the simvastatin dose.