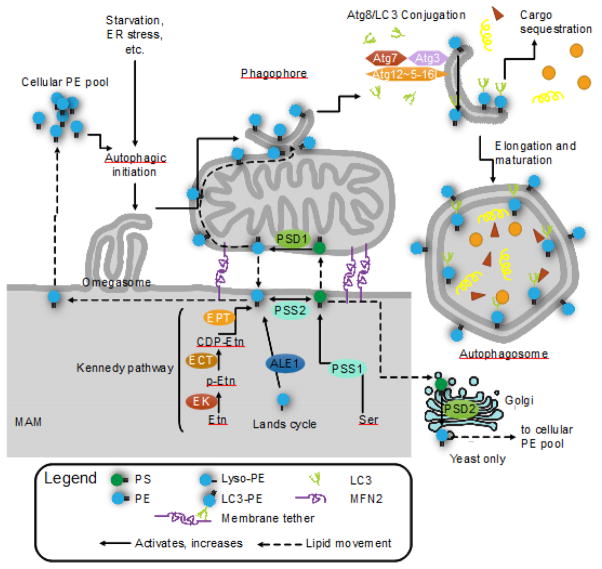
FIGURE 3. Mitochondrial phosphatidylethanolamine (PE) is required for autophagy.
PE is critical for the initiation of autophagy and elongation of autophagosomes. PE is synthesized through 4 pathways (5 for yeast): the CDP-ethanolamine/Kennedy pathway; baseexchange by phosphatidylserine synthase 2 (PSS2); re-acylation of lyso-PE via the Lands cycle (ALE1/LPLAT2); decarboxylation of phosphatidylserine (PS) by phosphatidylserine decarboxylase in the mitochondria (PSD1) or in the Golgi (PSD2, yeast only). PS is synthesized by PSS1, which catalyzes a base exchange with phosphatidylcholine (not pictured). The mitochondrial PSD1 and Kennedy pathway are the major sources of cellular PE. The cellular PE pool is divided between PE as a major component of most lipid bilayers, glycosylphosphatidylinositol (GPI) anchor synthesis and Atg8/LC3 lipidation. When cellular PE is limited, GPI anchor synthesis and Atg8/LC3 lipidation compete for substrates. Atg8/LC3 lipidation occurs on the phagophore, where Atg7 (E1), Atg3 (E2), and the Atg5 complex (Atg12~Atg5-Atg16L1) (E3-like) covalently link Atg8/LC3 onto PE, forming LC3-II. LC3-II is required selective autophagy and links autophagy receptors with an LC3-interacting region to the inner surface of the autophagosome. Knockdown of PSD1 significantly impairs autophagy and LC3 lipidation, indicating the importance of mitochondria-derived PE in autophagy over other sources. Exogenous ethanolamine (Etn) and PE can restore autophagy in PSD1 knockouts, and can stimulate autophagy and increase longevity in wild-type cells.