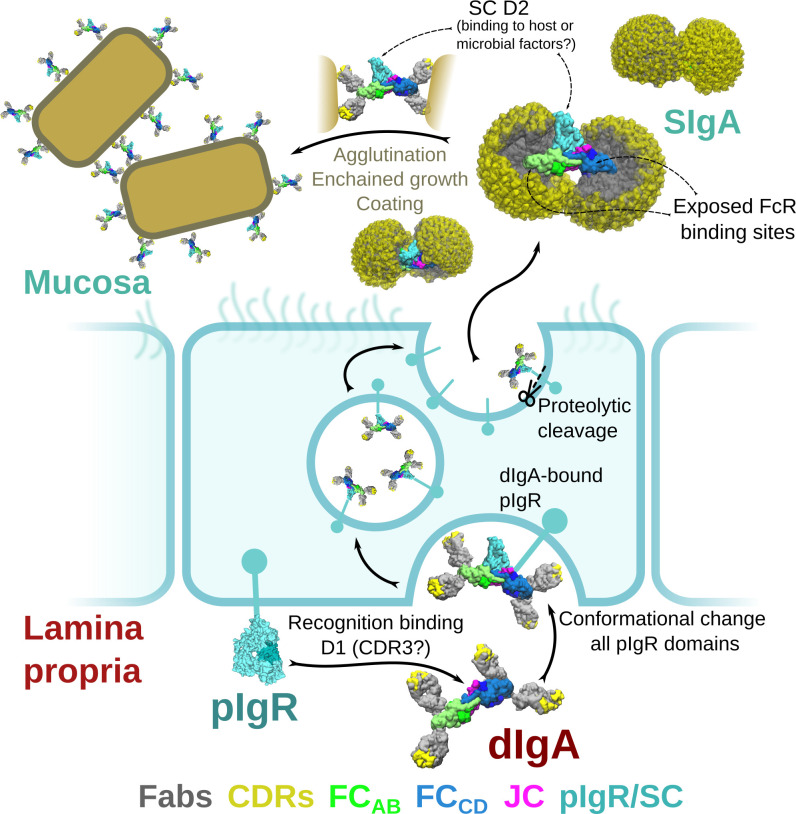
Figure 7. Model for the formation, transport,and function of SIgA.
Schematic summary depicting the unliganded SC structure (pdb code 5D4K) as pIgR bound to basolateral surface of an epithelial cell in its closed conformation and recognizing bent dIgA from the lamina propria. The pIgR binding to dIgA triggers a conformation change that repositions its domains to facilitate numerous stabilizing contacts with dIgA. The dIgA-pIgR complex transcytoses to the apical membrane where the pIgR is proteolytically cleaved, releasing SIgA into the mucosa. In the mucosa, SIgA Fabs (shown in all possible modeled positions) are directed toward the concave side of the antibody ‘looking’ for potential antigens while its Fc-receptor-binding regions are exposed on the convex side and accessible to potential host or microbial receptors. SC domains are also partially exposed; the D2 domain is almost completely accessible, protruding out of the SIgA where it may bind host and bacterial factors. Upon encountering antigen, SIgA Fabs bind, promoting antigen coating, agglutination, or enchained growth.