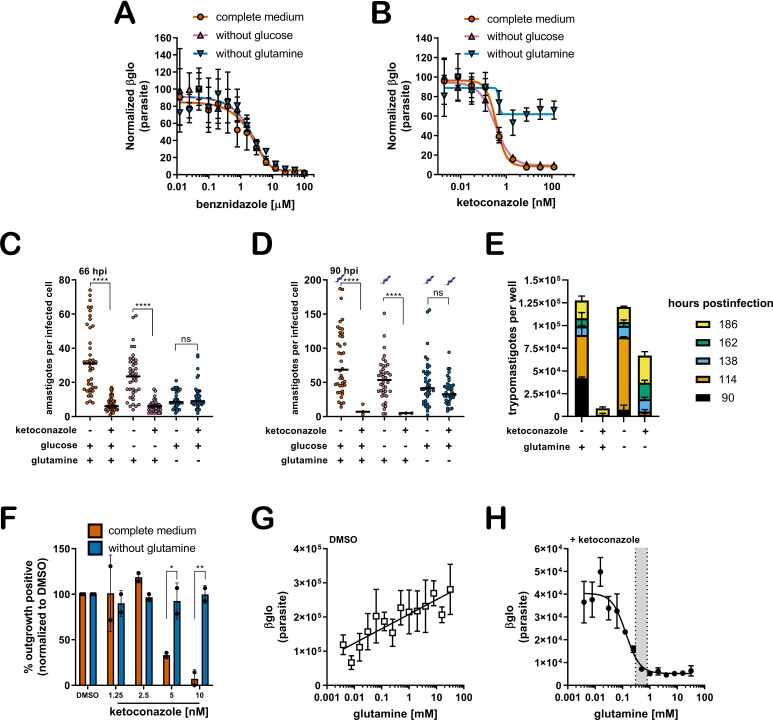
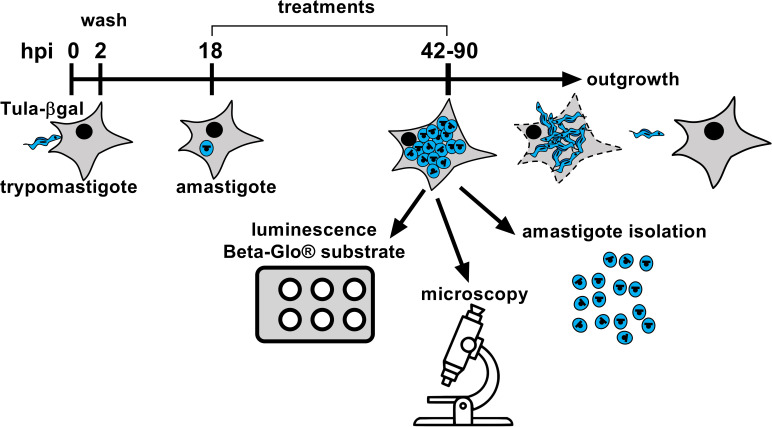
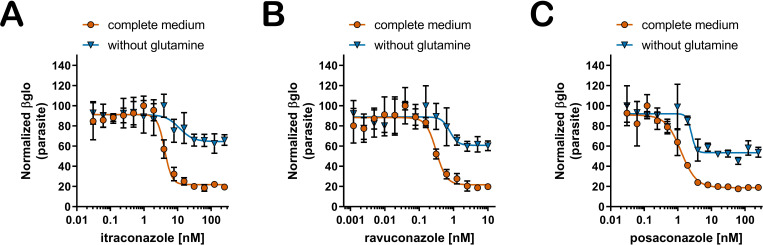
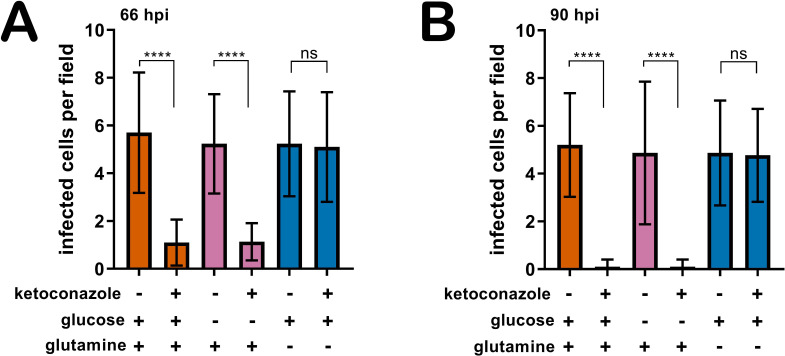
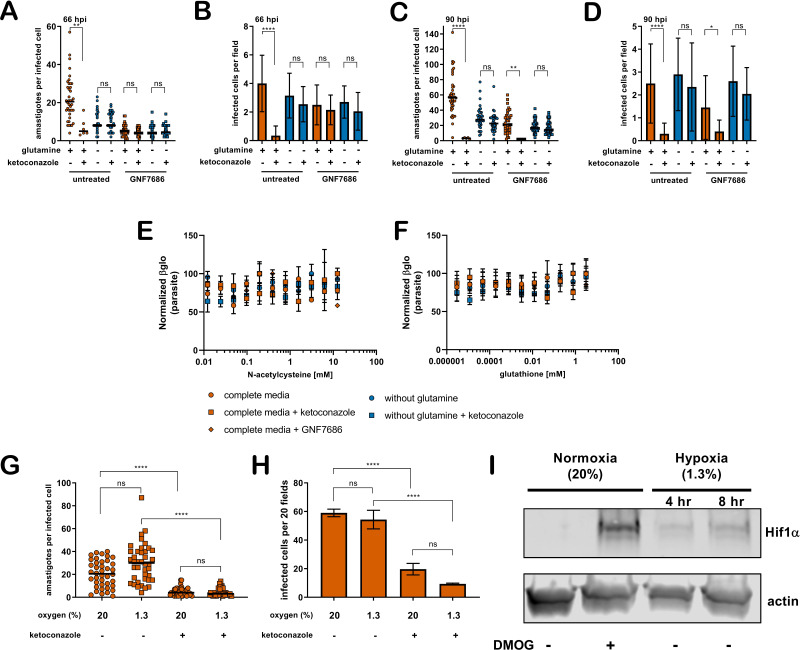
Figure 1. A lack of supplemental glutamine in growth medium protects intracellular T. cruzi amastigotes from the cytocidal effects of ketoconazole.
(A) Dose response curves at 66 hpi of benznidazole and (B) ketoconazole treatment, in the indicated media compositions, normalized to the largest mean in each treatment group. Mean (symbols) and standard deviation shown (n = 4). (C) Microscopic counts at 66 hpi and (D) 90 hpi of the number of amastigotes per infected host cell (n = 40), medians indicated. Cartoons at top of graph indicate conditions where extracellular trypomastigotes are visible in the culture supernatant. (E) Growth media was replaced and extracellular trypomastigotes were counted every 24 hr beginning at 90 hpi (n = 2). (F) Detection of clonal outgrowth 14 days after the indicated treatments, normalized to DMSO (vehicle) treatment. Mean and standard deviation shown, circles indicates values of two independent experiments with 28 wells used per treatment within an experiment. (G) Dose response curves of glutamine in the presence of DMSO or (H) ketoconazole (5 nM). Mean and standard deviation shown (n = 3). Grey shading indicates in panel I shows the physiological range found in human plasma (800–300 uM) (Cruzat et al., 2018). Statistical comparisons between medians (C,D) were performed using a Kruskal-Wallis test with Dunn’s multiple comparisons test (****p<0.0001, ns = not significant). Comparisons of means from outgrowth (F) was performed using a two-way ANOVA with Dunnett’s multiple comparisons test (*p<0.05, **p<0.01).