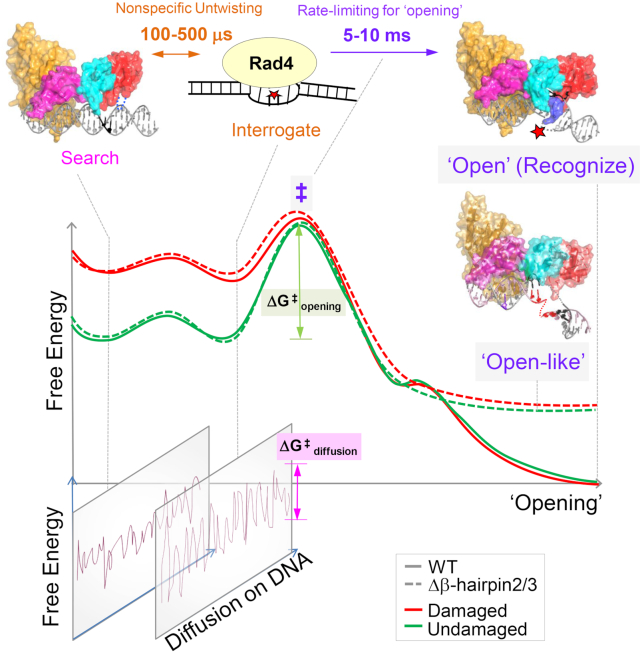
Figure 6.
Proposed DNA ‘opening’ trajectory and ‘kinetic gating’ mechanism of Rad4/XPC. The top panel illustrates distinct binding modes for Rad4/XPC as it searches for, interrogates, and recognizes a damaged site, and the time scales for fluctuations between these modes, based on prior studies (18–20). The middle panel shows a schematic free-energy profile along the ‘opening’ trajectory. The faster 100- to 500-μs nonspecific untwisting step entails a smaller energetic barrier than the slower 5- to 10-ms rate-limiting step (‡) of the ‘opening’ process. The rate-limiting step involves sufficiently unwound and bent DNA but with the nucleotides not yet fully or stably flipped out into the BHD2/BHD3 groove (19). The free energy barrier (ΔG‡opening) for ‘opening’ damaged DNA (red) is naturally lower than that for undamaged DNA (green) as DNA damage destabilizes the B-DNA structure. For Rad4 mutants that are lacking either β-hairpin2 or β-hairpin3, the protein can still overcome ΔG‡opening (although the barrier could be higher) to form ‘open-like’ structures that exhibit the same extent of unwinding as the fully ‘open’ structure with the WT (18), as monitored by the tCo-tCnitro FRET probes; however, the ‘open-like’ structures with the mutants are less stable and do not show well-resolved flipped-out nucleotides in the crystal structures (this study). MD simulations indicate that there can be more than one pathway that leads to ‘open-like’ or ‘open’ structures and demonstrate that the two β-hairpins function in a concerted manner to promote ‘opening’, but can also compensate for each other when one β-hairpin is lacking. The bottom panel illustrates that for each step along the ‘opening’ trajectory, there is also a kinetically competing process of diffusion of Rad4/XPC along the DNA, characterized by ΔG‡diffusion. For undamaged DNA, the high ΔG‡opening compared with ΔG‡diffusion favors the protein diffusing away before ‘opening’ a given site, while for damaged DNA this competition favors ‘opening’. However, when tethered, the diffusion of the protein is blocked and it can ‘open’ that site as long as the ΔG‡opening is thermally surmountable, suggesting that stalling of Rad4/XPC by another protein may be a mechanism employed by NER to enable the ‘opening’ of more resistant NER lesions.