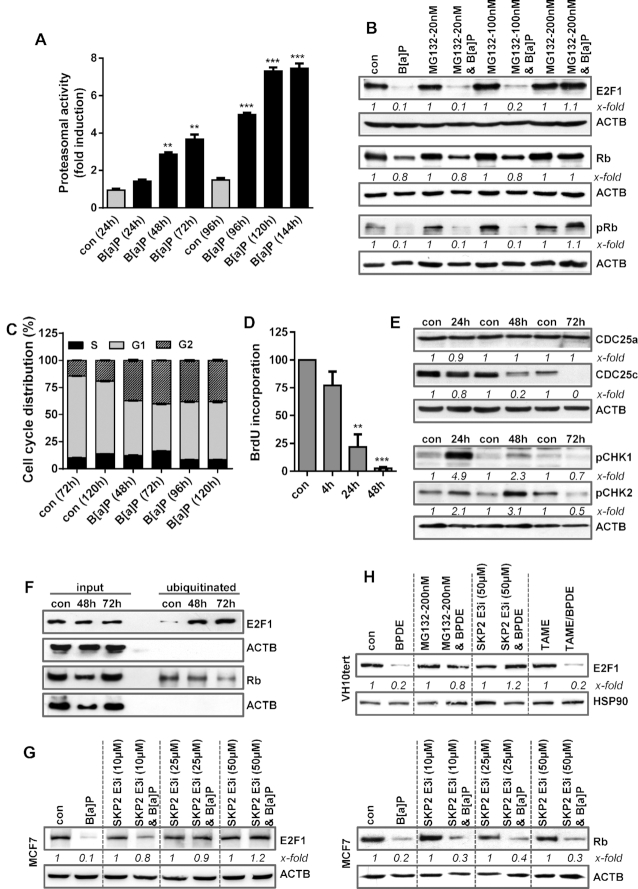
Figure 4.
(A) MCF7 cells were exposed to 1 μM B[a]P and proteasomal activity was measured. (B) MCF7 cells were treated with 1 μM B[a]P for 72 h in the presence or absence of the proteasomal inhibitor MG132. E2F1, Rb and pRb were detected by immunoblotting (C) Cell cycle distribution was measured in MCF7 cells using PI staining and flow cytometry 48–120 h after B[a]P exposure. (D) DNA synthesis was measured using BrdU incorporation 4–48 h after B[a]P exposure. (E) MCF7 cells were treated with 1 μM B[a]P. 24–72h later, the expression of CDC25a, CDC25c, pCHK1 and pCHK2 was detected by immunoblotting. (F) MCF7 cells were treated with 1 μM B[a]P in the presence of MG132. 48–72 h later, ubiqitinated proteins were isolated using the UBIQAPTURE-Q® kit and subjected to immuoblotting and the presence of E2F1 and Rb was detected. (G) MCF7 cells were treated with 1 μM B[a]P for 72 h in the presence or absence of a SKP2 inhibitor (SKP2 E3i). Expression of E2F1 and Rb was detected by immunoblotting. (H) VH10tert cells were treated with 0.25 μM BPDE for 72 h in the presence or absence of MG132, SKP2 E3i or TAME. E2F1 was detected by immunoblotting. (B, E–H) β-Actin was used as internal loading control. (A, C, D) Experiments were performed in triplicates and differences between treatment and control were statistically analyzed using Student's t test (non labelled = non-significant, *P< 0.1 **P< 0.01, ***P< 0.001). Concerning cell cycle distribution, at all time points a significant (*) decrease of cells in G1 and increase in the G2-phase was observed. (B, E, G, H) x-fold induction was measured densitometrically and is annotated under the respective blot.