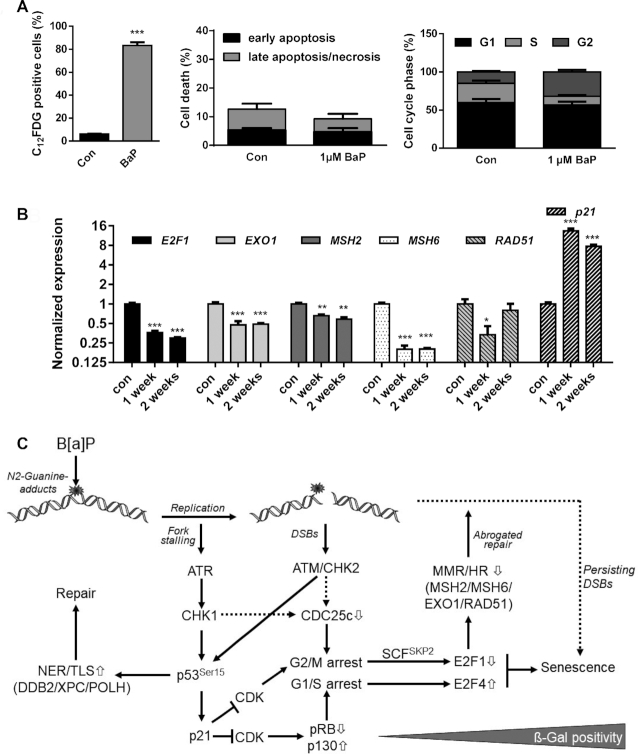
Figure 7.
(A) MCF7 cells were exposed to 1 μM B[a]P for one week. Senescence was measured by C12FDG staining (left graph), cell death by Annexin V/PI staining (middle graph) and cell cycle distribution by PI staining (right graph). (B) MCF7 cells were exposed to 1 μM B[a]P for one or two weeks. Expression of E2F1, EXO1, MSH2, MSH6, RAD51 and p21 was detected by qPCR. (A, B) Experiments were performed in triplicates and differences between treatment and control were statistically analyzed using Student's t test (non-labelled = non significant, *P< 0.1 **P< 0.01, ***P< 0.001). (C) Model explaining the interaction between E2F1 signalling abrogation, transcriptional repression of DNA repair and induction of senescence. B[a]P induces genotoxic stress, which activates the DDR resulting either from replicative stress or the formation of DSBs. Activation of ATR, ATM, CHK1 and CHK2 leads to phosphorylation and activation of p53 and, subsequently, to enhanced expression of DNA repair genes DDB2, XPC and POLH and the CDK inhibitor p21. p21 blocks E2F1 activation and activates the DREAM complex, which arrests cells in G1/S and G2/M. In parallel, activation of the DDR triggers degradation of CDC25c, which contributes to the G2/M arrest. In G2-arrested cells, E2F1 is ubiquitinated and degraded by the proteasome, whereas in G1-arrested cells the DREAM complex blocks E2F1 transcription. The dual inhibition of the E2F1 signalling pathway represents the initial step in senescence induction and, in addition, supresses MSH2, MSH6, EXO1 and RAD51, which therefore represents an early mark for B[a]P-induced senescence. It is to suppose that the senescence-associated inhibition of DNA repair further blocks the repair of critical DNA lesions, and therefore might be involved in the maintenance of senescence.