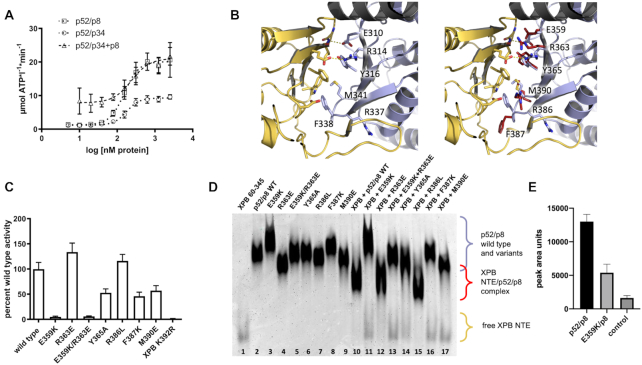
Figure 2.
Activation of XPB’s ATPase by p52/p8 and interaction studies of ctp52 MD2 and ctXPB NTE. (A) ATPase activity profile of ctXPB activated by increasing amounts of different ctp52 complexes. Ctp52 complexes were titrated to a fixed amount of ctXPB. The x-axis shows the logarithmic concentration of the respective ctp52 complex. The y-axis shows the NADH-consumption-derived ATP turnover rate in μmol ATP per liter per minute. N ≥ 3. (B) Structural insight into the human p52 (light blue) – XPB (yellow) interface taken from 6NMI. On the left hand side, key residues in the interface are labeled. On the right hand side, the corresponding residues in ctp52 (red) are superimposed to the human counterparts and labeled. The depicted ctp52 residues were mutated and analysed with respect to their influence on ctXPB’s ATPase activity (C) and in terms of their interaction with the ctXPB NTE (D). (C) Effect of ctp52/ctp8 wild type and variants on the ATPase activity of ctXPB. N = 8. (D) Native PAGE analysis of the interaction between the ctp52/ctp8 variants and ctXPB NTE (ctXPB 60–345). Individual proteins are shown in lanes 1–9. Ctp52 wild type and variants in complex with ctp8 and ctXPB NTE are shown in lanes 10–17. (E) Pull-down of ctp52/ctp8 and ctp52_E359K/ctp8 with Streptavidin-tagged ctXPB as bait. The pull down fractions were loaded on SDS PAGEs and the SDS PAGE band strength of p52/p8 wild type and E359K was analysed with ImageJ to quantify the interaction capability with full length XPB. Mean values with SD were derived with GraphPad Prism. To exclude unspecific binding to the beads, p52/p8 was loaded as a control to the beads without XPB. N = 3.