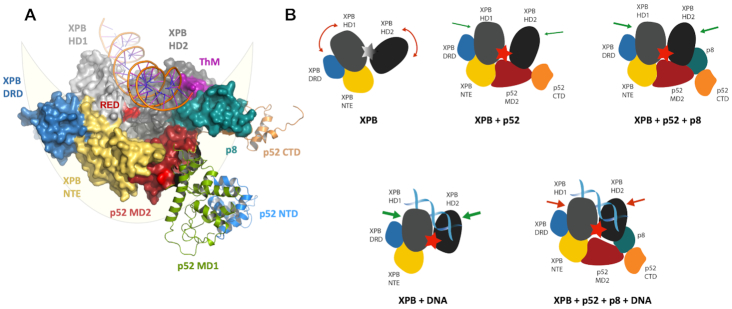
Figure 5.
Model for p52/p8 mediated activation and restriction of XPB’s ATPase. (A) Structural model of the lunate-like ring that encircles the two XPB helicase domains. XPB comprises the following domains: NTE (yellow), DRD domain (blue), HD1 (light gray) and HD2 (dark gray). The ThM and RED motifs are highlighted in purple and red, respectively. P52 comprises NTD (blue), MD1 (green), MD2 (red), and CTD (orange). P8 is depicted in mint green, the DNA duplex in orange. The lunate-like ring that encircles the XPB helicase domains is schematically depicted in light yellow. Domains participating in the lunate-like ring are shown as solid surfaces. P52 domains not participating in the lunate-like ring are shown as cartoon. The structural model was derived from Schilbach, et al. (27) and our p52 structures. (B) Model of the differential XPB activation by DNA, p52/p8, p52 or p52/p8/DNA. If no activator is present, the XPB helicase domains HD1 and HD2 are too flexible and ATP hydrolysis cannot be performed. P52 binding fixes the helicase domains and thus brings them into close proximity to each other enabling ATP hydrolysis. Binding of p8 further extends the lunate-like ring and further restrains the flexibility of the two helicase domains resulting in an even higher activity. When DNA binds to XPB, the helicase domains are also restrained and brought into close proximity, leading to the highest ATPase activity. Simultaneous p52 or p52/p8 and DNA binding also results in an activation of XPB, but the activation level is limited due to the reduced flexibility of XPB.