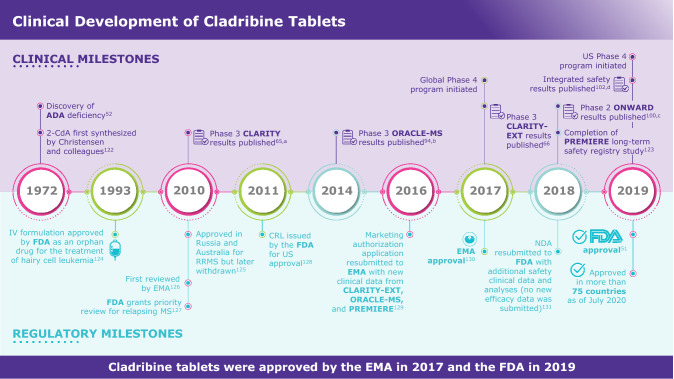
Fig. 1.
Cladribine tablets development milestones [51, 52, 65, 66, 94, 100, 102, 122–131]. aNaive and treatment-experienced patients with RRMS. bTreatment-naive patients at high risk for developing MS. cPatients with active RRMS in combination with IFN-β. dIncluding patients from CLARITY, CLARITY–EXT, ORACLE-MS, and PREMIERE. ADA adenosine deaminase, 2-CdA 2-chlorodeoxyadenosine deaminase, CRL complete response letter, EMA European Medicines Agency, FDA Food and Drug Administration, IFN interferon, IV intravenous, MS multiple sclerosis, NDA new drug application, RRMS relapsing–remitting MS