Abstract
Cytomegalovirus (CMV) is a double-stranded DNA virus, which infects a large portion of the adult population. In immunocompetent patients, it typically is asymptomatic or manifests as mild and self-limiting flu-like illness symptoms, whereas in immunocompromised patients, CMV can cause significant disease. Herein we report an unusual case of CMV pancreatitis in an immunocompetent 75-year-old female. Patient developed severe significant pancreatic necrosis that failed non-operative management, and ultimately underwent pancreatic necrosectomy. Later on, she developed three spontaneous gastric perforations. The first two perforations were managed operatively, but after the third perforation family decided not to undergo another operation. The CMV pancreatitis diagnosis was based on pancreatic histopathology and confirms by a prompt response to ganciclovir. Patient was promptly started on intravenous (IV) ganciclovir which resulted in clinical recovery and she remained asymptomatic more than one-year post op. This is a rare case of CMV pancreatitis with gastric perforations in an immunocompetent patient. High degree of suspicion and appropriate treatment are important for such clinical scenarios.
Abbreviations: CMV, cytomegalovirus; CKD, chronic kidney disease; BMI, body mass Index; CT, computed tomography; OR, operating room; ICU, intensive care unit; POD, post-operative day; PCR, polymerase chain reaction; EGD, esophagogastroduodenoscopy; AST, aspartate transferase; ALT, alanine transferase; EBV, Epstein Barr virus; DIC, disseminated intravascular coagulation; GI, gastrointestinal
Keywords: Pancreatitis, Cytomegalovirus, Pancreatic necrosis, Gastric perforation, Ganciclovir
Introduction
Cytomegalovirus or human herpes virus (HHV-5) belongs to Herpesviridae family and forms characteristic intra-nuclear inclusion bodies. Initially, German scientists noticed these inclusion bodies in 1881 and later, Weller, Smith and Rowe independently isolated and grew CMV from man and mice in 1956−57 [1]. CMV infection in immunocompetent subjects is usually asymptomatic and viral shedding can happen in saliva, breast milk, uterine, cervical secretions and semen [1]. According to The National Health and Nutrition Examination Surveys (NHANES) in the period of 1988–2004, CMV seroprevalence in United States ranged from 32 %–90 %. CMV seropositivity was independently associated with older age, female sex, foreign birthplace, low socioeconomic status, high household crowding and low education level [2]. CMV infection in immunocompetent hosts is usually a benign, self-limiting, viral-like syndrome that may manifest as a mononucleosis like illness [3]. On the contrary, CMV can cause significant morbidity and mortality in immunocompromised patients, especially HIV patients, organ transplant recipients, and newborns [2,3].
Case presentation
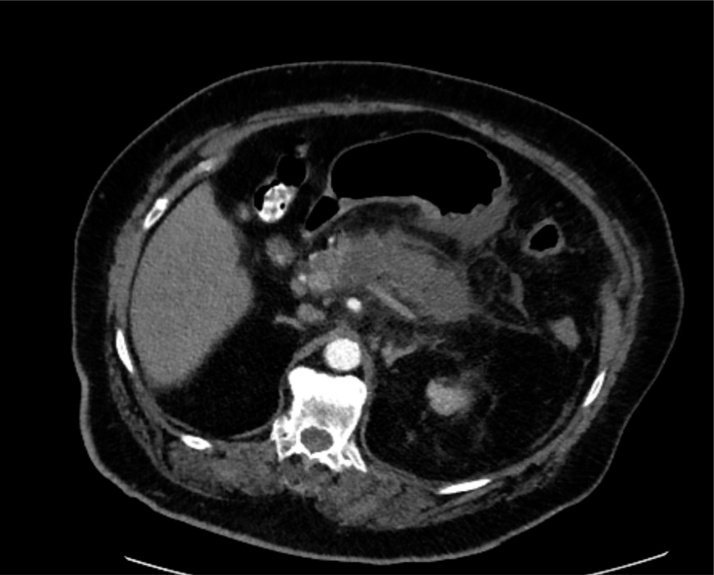
The patient was a 75-year-old female with past medical history significant for hypertension, diabetes mellitus type II, chronic kidney disease (CKD stage II), obesity (BMI 38.4 kg/m2) and hyperlipidemia who was transferred from an outside facility with a diagnosis of gallstone pancreatitis. She had experienced right upper quadrant abdominal pain for almost two weeks associated with nausea, and vomiting. On admission, she was afebrile with stable vital signs; however, her abdominal exam was notable for epigastric tenderness. The patient’s laboratory work was significant for white blood cell count of 22,200/μL, serum lipase of 804 U/L and amylase level of 484 U/L. Computed tomography (CT) of the abdomen at the time of admission showed a small gallstone in gallbladder without any signs of cholecystitis, or intra or extrahepatic biliary ductal dilatation, as well as greater than 75 % necrosis of pancreas with surrounding inflammatory changes as well as non-occlusive splenic vein thrombosis (Fig. 1).
Fig. 1.
CT scan with IV contrast demonstrating pancreatic necrosis with surrounding inflammation.
Patient’s lipid profile was within normal limits while on lipid lowering therapy and her calcium level was in normal range. She drank alcohol occasionally and was not taking any medications with side effect of pancreatitis. The CT scan did not show any anatomic anomaly and patient also denied any recent gastrointestinal procedure.
Patient was treated with intravenous fluid, IV antibacterials, analgesics and bowel rest. Her serum lipase and amylase level started improving; however, she continued to experience persistent leukocytosis with inability to tolerate oral intake and progressed to hypoxemic respiratory failure requiring intubation and mechanical ventilation. Due to lack of clinical improvement and persistent leukocytosis, a repeat CT scan abdomen/pelvis with contrast was performed and showed a large collection (13 × 19 × 23 cm) with no active bleeding or signs of infection. Patient was taken to the operating room (OR) for pancreatic necrosectomy, abdominal washout and wide drainage. Postoperatively, she was transferred to the surgical intensive care unit for vasopressor requirements and ventilator support. On post-operative day (POD) 10, a repeat CT scan of abdomen/pelvis was performed due to persistent leukocytosis despite patient being on antimicrobial and anti-fungal therapy and it showed a posterior wall gastric perforation (Fig. 2A). Patient was taken back to the OR and the gastric perforation was repaired primarily in two layers. Patient’s vasopressor requirements decreased, but she required continuous close monitoring in the surgical ICU. One week after gastric perforation repair, patient had another febrile episode with elevated WBC count, and another posterior wall gastric perforation was discovered on CT imaging (Fig. 2B). Patient was taken back to the OR for primary repair of the second gastric perforation and abdominal washout. We also performed more pancreatic necrosectomy on both subsequent surgeries.
Fig. 2.
A: Initial CT scan demonstrating gastric perforation in the lesser sac. B: CT scan demonstrating second perforation in greater curvature of stomach.
Twenty-eight days later from index operation, patient developed a third posterior wall gastric perforation. After detailed discussion with family, they elected not to pursue an additional surgery at that time. Her pancreatic histopathology report from last operation showed pancreatic necrosis with clusters of cells with CMV inclusion bodies on Hematoxylin and Eosin (H&E) and CMV antibody stains (Fig. 3).
Fig. 3.
Histology slides demonstrating A: necrotic pancreas (black arrow) and cluster of CMV cells (yellow arrow) slides and B: positive immune stain for CMV (black arrow).
At this point we checked patient’s HIV status and it was negative. We immediately initiated intravenous ganciclovir 200 mg IV every 12 h. Lab work at this point, revealed negative CMV IgM titer, positive CMV IgG titer and a CMV PCR of 3,860,104 IU/mL. After initiation of ganciclovir, she started to clinically improve, and her leukocytosis started trending down. Patient was able to be weaned off vasopressors. Her CMV PCR titer decreased to 259 IU/mL after 14 days of ganciclovir treatment. Other supportive care consisted of total parenteral nutrition (TPN) tracheostomy and a jejunostomy feeding tube placement. Ganciclovir was discontinued after 16 days of therapy. Her hospital course was later complicated with abdominal wound dehiscence for which she underwent debridement, negative pressure wound therapy and skin grafting later. Of note, her urine and bronchoalveolar lavage cultures were positive for Candida albicans and Pseudomonas aeruginosa, which were treated appropriately. Patient was discharged to a rehabilitation center initially then home in a stable condition. Her tracheostomy was decannulated, and she was gradually started on regular diet with no evidence of leak on follow up CT scans. Five months later, an outpatient esophagogastroduodenoscopy (EGD) was performed, which were negative for any malignancy, Helicobacter pylori and CMV infection.
Discussion
Although clinically significant CMV infection is considered rare in immunocompetent individuals, there are multiple reports in literature highlighting that it should be considered in differential diagnoses [[3], [4], [5], [6], [7], [8], [9]]. Multiple organ systems can be affected by CMV, the most common being gastrointestinal tract with manifestations such as colitis, gastritis, gastric ulcer [10,11], hepatitis [12], pancreatitis [[12], [13], [14], [15]], duodenitis, or enteritis. Other organ systems affected by CMV include central nervous system (meningitis, encephalitis, and transverse myelitis), hematological disorders (hemolytic anemia, thrombocytopenia), vascular thrombosis (venous thromboembolism, portal vein thrombosis), and ocular involvement (uveitis) and lung disease (pneumonitis).
After primary exposure, there are three different CMV manifestations in a human host: latent CMV, CMV infection and CMV disease. Latent CMV refers to presence of CMV viral DNA within the human host cells without detectable active replication. CMV infection refers to evidence of active viral replication without symptoms and CMV disease refers to CMV infection with overt symptoms [8]. Primary CMV infection in an immunocompetent patient manifests as flu-like symptoms or mononucleosis-like syndrome, consisting of prolonged fever, myalgia, malaise with less than 5% patients presenting with jaundice. Liver function tests (AST, ALT, total bilirubin and alkaline phosphatase) are usually three times of the upper normal limit and in rare cases they can be elevated to five times the upper limit. Diagnosis of CMV mononucleosis-like syndrome is made after exclusion of Epstein-Barr virus (EBV) mononucleosis. Other supportive lab results for diagnosis are lymphocytosis (>4000/μL), CMV IgM titer (positive) and a 4-fold increase in IgG titers within 2 weeks. Additional evidence of active CMV replication includes positive pp65 antigenemia (>10/250,000 leukocytes) or a serum CMV PCR > 104 copies/mL of whole blood [5,8]. In immunocompetent patients, CMV disease generally is self-limited, but severe disease is associated with critical illness. A Swedish case series showed 3.4 % of ICU patients with severe CMV disease had multi-organ involvement (>2 organ systems) [5,8].
On a literature search, and to the best of our knowledge, we were able to find only four case reports with documented pancreatitis and CMV infection in immunocompetent patients [13,[15], [16], [17]]. Chan, et al., diagnosed CMV pancreatitis based on a tissue sample after pancreatoduodenectomy done for biliary stricture. The CMV serology was IgM (negative), IgG (positive) and CMV PCR (negative) and the patient was treated with ganciclovir [12]. Oku, et al., reported a case of CMV pancreatitis in a patient with pancreatic divisum and distal bile duct stricture, leading to pancreatoduodenectomy to rule out malignancy due to inconclusive work up. Diagnosis was confirmed by observing inclusion bodies in the ductular or acinar cells and patient was not treated with antiviral medications [14]. Yasumoto, et al. described a case of systemic CMV disease triggering pancreatitis followed by diabetic ketoacidosis, rhabdomyolysis and acute kidney failure. CMV infection was confirmed serologically as well as by infiltration of T cell lymphocytes predominantly in her muscles and thrombocytopenia without any disseminated intravascular coagulation (DIC). There were no inclusion bodies found either in muscle or renal biopsy specimens [13]. Sakakibara, et al., reported a case of acute pancreatitis caused by CMV associated duodenal papillitis [15]. In last two case reports there was no pancreatic tissue biopsy specimen.
CMV pancreatitis diagnosis is more difficult to establish than other CMV infection in gastrointestinal (GI) tract. Biopsy of pancreas is generally not recommended. In our case, diagnosis was made based on the pathology report, and our patient also had CMV IgG and PCR positive results.
Gastric perforations in general can be secondary to peptic ulcer disease, malignancy, trauma, radiation, infection vascular and iatrogenic causes. We presume recurrent gastric perforations in our patient was due to pancreatic enzymatic digestion of the posterior stomach wall. Although conclusive diagnosis of gastric CMV involvement could not be made without a gastric wall biopsy in our case but retrospectively, our patient clinically improved after antiviral treatment and did not develop any further perforations. Stomach and esophagus biopsy done at a later date were negative for CMV and malignancy, but those samples were obtained after ganciclovir treatment.
An underestimated complication of CMV infection is venous/portal thrombosis which was seen in our patient. A review article from Abgueguen, et al. found 19 reported cases of venous thrombosis and pulmonary embolism in immunocompetent patient with active CMV injection. There are several proposed mechanisms for CMV induced-vascular thrombosis described by Abgueguen, et al. [16].
The diagnosing and predicting complications of CMV disease can be challenging, but there are several reviews and case reports discussing CMV diagnosis. Al-Omari, et al. provided a summary of studies published regarding CMV disease in ICU patients from 1990 to 2015 and discussed challenges in diagnosis and developing a predictive model for CMV disease in critical care setting [17]. Kanno, et al. also published a series of seven patients with histopathologically proven CMV disease, but none of them had pancreatitis [18]. Von Müller, et al. shared their prospective study results of 25 immunocompetent CMV-seropositive patients with septic shock with > 7 days of ICU stay. Many factors can stimulate CMV reactivation in patients with septic shock, e.g. pro-inflammatory cytokines, transient immune paralysis and medications [19]. Despite the difficulty in diagnosing and predicting CMV complications, when treated, patients have good outcomes. Guidelines in immunocompromised patients, recommend either IV ganciclovir or oral valganciclovir for the treatment of CMV infections. Ganciclovir, which was used in this case, is to be dosed as 5 mg/kg every 12 h with normal renal function [20]. Evidence supporting the use of ganciclovir for the treatment of CMV infections in immunocompetent patient was presented by Eddleston, et al. in 1997 [21]. In the absence of multicenter trial, initiation of antiviral treatment is the treating physician’s judgement and our literature search supported better outcomes in immunocompetent patients who received treatment as in our case too [[17], [18], [19],21].
Conclusion
CMV is reported to cause self-limited disease in immunocompetent patients. Pancreatitis and pancreatic necrosis secondary to CMV is rare; however, it should be considered in the differential diagnosis if a patient is experiencing symptoms of systemic inflammation unresponsive to standard of care. Non-invasive test such as serum PCR can aid in establishing diagnosis but in some cases tissue biopsy may be needed. Based on extensive literature search, this is the first reported case of CMV pancreatitis in an immunocompetent patient resulting in pancreatic necrosis and possible recurrent gastric perforations. Treatment with ganciclovir and necrosectomy played a significant role in patient recovery.
Declaration of Competing Interest
The authors report no declarations of interest.
Consent
Written informed consent for publication of clinical details, and images was obtained from the patient.
Author contributions
MS: Patient management, data collection, extensive literature review, drafting and editing.
AD: Patient management, data collection, initial draft, literature review.
ON: Provided histopathological data and figures.
RS: Critical review of manuscript, editing and feedback.
IG: Critical review of manuscript, editing and feedback.
RK: Critical review of manuscript, editing and feedback.
Author statement
In preparation of this case report, Muhammad I. Saeed, Atbin Doroodchi were involved in patient management, data collection, literature review, as well as drafting and editing the manuscript. Okechukwu Nwogbo was involved in providing and analyzing histopathological data. Imran Gani, Rajan Kapoor, and Rachel Stephens participated in critical review of manuscript, and its editing. The manuscript was reviewed by all authors listed.
Acknowledgement
Special Thanks to Melissa Laub Pharm D for proofreading this manuscript.
References
- 1.Ho M. The history of cytomegalovirus and its diseases. Med Microbiol Immunol. 2008;197(2):65–73. doi: 10.1007/s00430-007-0066-x. [DOI] [PubMed] [Google Scholar]
- 2.Bate S.L., Dollard S.C., Cannon M.J. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafailidis P.I., Mourtzoukou E.G., Varbobitis I.C., Falagas M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J. 2008;5:47. doi: 10.1186/1743-422X-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz C.A., Henle W., Henle G., Snover D., Rudnick H., Balfour H.H., Jr. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65(2):124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Orasch C., Conen A. Severe primary cytomegalovirus infection in the immunocompetent adult patient: a case series. Scand J Infect Dis. 2012;44(12):987–991. doi: 10.3109/00365548.2012.697637. [DOI] [PubMed] [Google Scholar]
- 6.Nolan N., Halai U.A., Regunath H., Smith L., Rojas-Moreno C., Salzer W. Primary cytomegalovirus infection in immunocompetent adults in the United States – a case series. IDCases. 2017;10:123–126. doi: 10.1016/j.idcr.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nangle S., Mitra S., Roskos S., Havlichek D. Cytomegalovirus infection in immunocompetent adults: Is observation still the best strategy? IDCases. 2018;14 doi: 10.1016/j.idcr.2018.e00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakhreddine A.Y., Frenette C.T., Konijeti G.G. A practical review of cytomegalovirus in gastroenterology and hepatology. Gastroenterol Res Pract. 2019;2019 doi: 10.1155/2019/6156581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaemsupaphan T., Limsrivilai J., Thongdee C., Sudcharoen A., Pongpaibul A., Pausawasdi N. Patient characteristics, clinical manifestations, prognosis, and factors associated with gastrointestinal cytomegalovirus infection in immunocompetent patients. BMC Gastroenterol. 2020;20(1):22. doi: 10.1186/s12876-020-1174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebisutani C., Kawamura A., Shibata N., Nasu M., Ueno R., Mimura K. Gastric ulcer associated with cytomegalovirus in an immunocompetent patient: method for diagnosis. Case Rep Gastroenterol. 2012;6(2):365–368. doi: 10.1159/000339714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krajicek E., Shivashankar R., Hansel S. Cytomegalovirus and the seemingly immunocompetent host: a case of a perforating gastric ulcer. ACG Case Rep J. 2017;4:e27. doi: 10.14309/crj.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan A., Bazerbachi F., Hanson B., Alraies M.C., Duran-Nelson A. Cytomegalovirus hepatitis and pancreatitis in the immunocompetent. Ochsner J. 2014;14(2):295–299. [PMC free article] [PubMed] [Google Scholar]
- 13.Yasumoto N., Hara M., Kitamoto Y., Nakayama M., Sato T. Cytomegalovirus infection associated with acute pancreatitis, rhabdomyolysis and renal failure. Intern Med. 1992;31(3):426–430. doi: 10.2169/internalmedicine.31.426. [DOI] [PubMed] [Google Scholar]
- 14.Oku T., Maeda M., Waga E., Wada Y., Nagamachi Y., Fujita M. Cytomegalovirus cholangitis and pancreatitis in an immunocompetent patient. J Gastroenterol. 2005;40(10):987–992. doi: 10.1007/s00535-005-1683-z. [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara Y., Nakazuru S., Kodama Y., Mita E. Acute pancreatitis caused by cytomegalovirus-associated duodenal papillitis. Ann Gastroenterol. 2018;31(1):122. doi: 10.20524/aog.2017.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abgueguen P., Delbos V., Ducancelle A., Jomaa S., Fanello S., Pichard E. Venous thrombosis in immunocompetent patients with acute cytomegalovirus infection: a complication that may be underestimated. Clin Microbiol Infect. 2010;16(7):851–854. doi: 10.1111/j.1469-0691.2009.03022.x. [DOI] [PubMed] [Google Scholar]
- 17.Al-Omari A., Aljamaan F., Alhazzani W., Salih S., Arabi Y. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann Intensive Care. 2016;6(1):110. doi: 10.1186/s13613-016-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanno M., Chandrasekar P.H., Bentley G., Vander Heide R.S., Alangaden G.J. Disseminated cytomegalovirus disease in hosts without acquired immunodeficiency syndrome and without an organ transplant. Clin Infect Dis. 2001;32(2):313–316. doi: 10.1086/318449. [DOI] [PubMed] [Google Scholar]
- 19.von Muller L., Klemm A., Weiss M., Schneider M., Suger-Wiedeck H., Durmus N. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razonable R.R., Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13512. [DOI] [PubMed] [Google Scholar]
- 21.Eddleston M., Peacock S., Juniper M., Warrell D.A. Severe cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 1997;24(1):52–56. doi: 10.1093/clinids/24.1.52. [DOI] [PubMed] [Google Scholar]