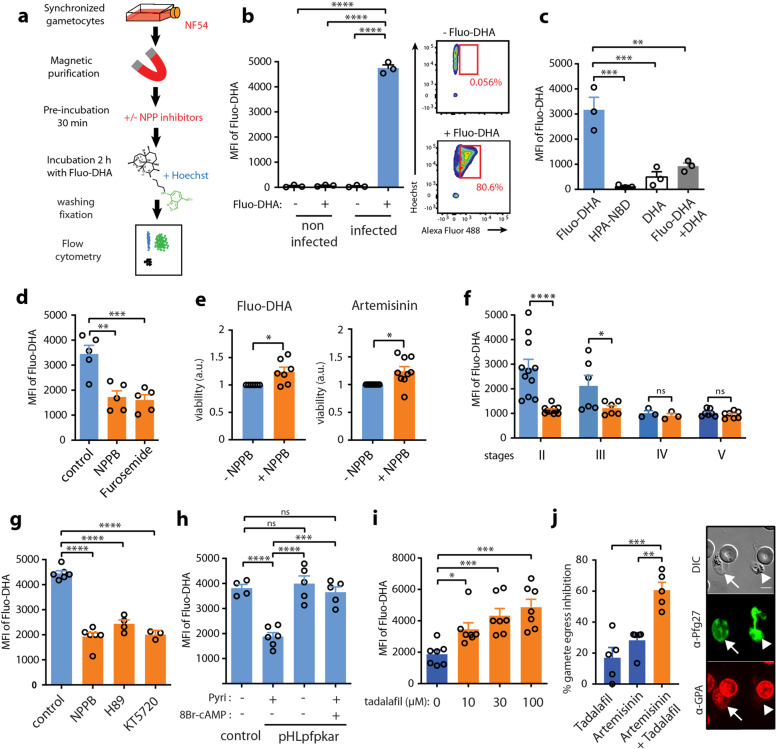
Fig. 4. NPPs contribute to the uptake of artemisinin derivatives.
a Diagram illustrating the uptake assay. b Left: Quantification of Fluo-DHA uptake in uninfected erythrocytes and in early GIE by flow cytometry. Right: scatter plots showing the gating strategy for Fluo-DHA uptake. c Competition assay between Fluo-DHA and DHA, and control of HPA-NBD and DHA-induced fluorescence levels. d Inhibition of Fluo-DHA uptake in early GIE upon 100 µM NPPB or Furosemide incubation. e Viability (luciferase activity) of early gametocytes of the NF54-cg6-pfs16-CBG99 line 48 h after a 3-h incubation with 150 nM Fluo-DHA or 5 µM artemisinin, with or without 100 µM NPPB. The graph shows the ratio of luciferase activity (drug-treated/control) and is normalized to the condition without NPPB. a.u. arbitrary units. f Quantification of Fluo-DHA uptake during gametocytogenesis with (orange) or without (blue) 100 µM NPPB. g Fluo-DHA uptake in early GIE upon 100 µM H89 or 10 µM KT5720 incubation. h Fluo-DHA uptake in early GIE from NF54 (Control) and the transgenic pHLpfpkar line, cultivated with or without pyrimethamine (Pyri), or preincubated with 100 µM 8Br-cAMP. i Fluo-DHA uptake in stage V GIE upon 0, 10, 30 and 100 µM tadalafil incubation. j Left: % inhibition of gamete egress after a 24-h incubation with 5 µM artemisinin, with or without 30 µM tadalafil. Right: gamete egress observed by IFAs. Samples were co-stained with mouse anti-glycophorin A (GPA, red) and rabbit anti-Pfg27 (green) IgG. DIC differential interference contrast. Arrow: mature GIE with an intact erythrocyte membrane, arrowhead: egressed gamete. Scale bars: 5 μm. Circles indicate the number of independent experiments and error bars show the SEM. Statistical significance is determined by one-way ANOVA with Dunnet correction (b−d, g, i) or with Sidak correction (f, h, j) for multiple comparisons or by a Mann−Whitney test (e). ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns: non-significant difference.