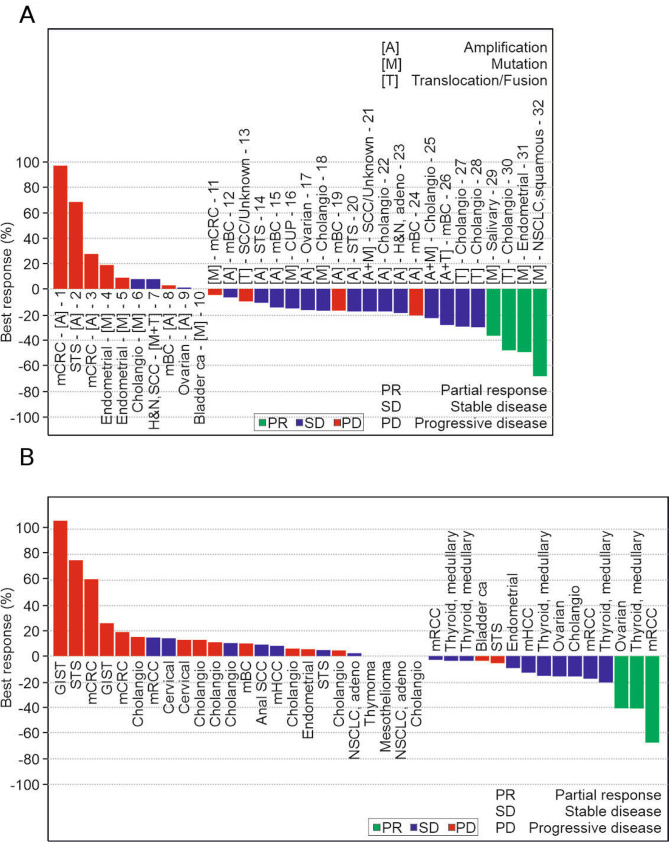
Figure 3.
ODM-203 best tumour response (RECIST) for FGFR patients (A) and non-FGFR patients (B). ITT 76 patients, 4 patients with low exposure (100–200 mg) and one patient with non-evaluable non-target lesions (600 mg) are not included. Unscheduled visits are included in the data. Transcript of abbreviations: CUP, cancer of unknown primary; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stromal tumour; H&N, head and neck; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; mHCC, metastatic hepatocellular carcinoma; NSCLC, non-small cell lung cancer; RECIST, Response Evaluation Criteria in Solid Tumours; SCC, squamous cell carcinoma; STS, soft-tissue sarcoma.