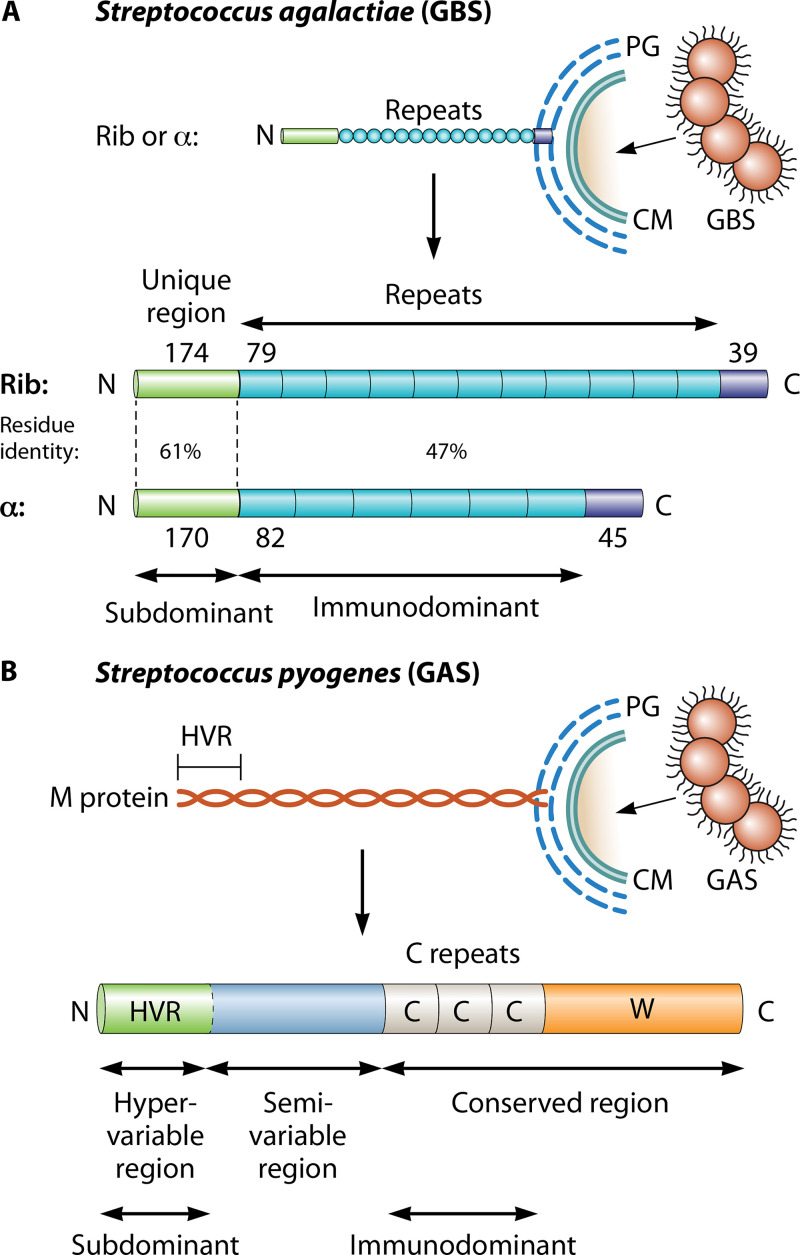
FIG 3.
Subdominant domains in streptococcal surface proteins. (A) Schematic of the surface-exposed forms of the Rib and α proteins of Streptococcus agalactiae (GBS) (79, 80). These two proteins are the most common members of a family of elongated and highly repetitive streptococcal proteins. Each protein has a surface-distal amino-terminal domain, a region with long repeats, and a short carboxy-terminal region that promotes covalent linkage to the bacterial peptidoglycan (PG) layer, located outside the cell membrane (CM). The number of amino acid residues in a region (or repeat) is indicated. While all repeats are completely identical within Rib or α, they vary in number among clinical isolates and are different in Rib and α but show 47% residue identity. The amino-terminal domains are strikingly subdominant but are targets for protective antibodies (17). (B) Schematic of the surface-exposed form of Streptococcus pyogenes M protein. All strains of S. pyogenes express an M protein, encoded by the emm gene (95). This fibrillar coiled-coil protein has an amino-terminal hypervariable region (HVR) of ∼50 to 100 amino acid residues, a conserved carboxy-terminal region that includes C repeats (each with 35 or 42 residues), and a wall-spanning region (W). The HVR exhibits extreme sequence divergence among strains but is stable within a strain and represents a distinct domain that in many (but not all) M proteins specifically binds a human complement regulator. The central part of M protein is also variable among strains and typically includes domains that bind human plasma proteins, e.g., fibrinogen or IgA (95). While the HVR is the major target for protective antibodies, it is strikingly subdominant (18).