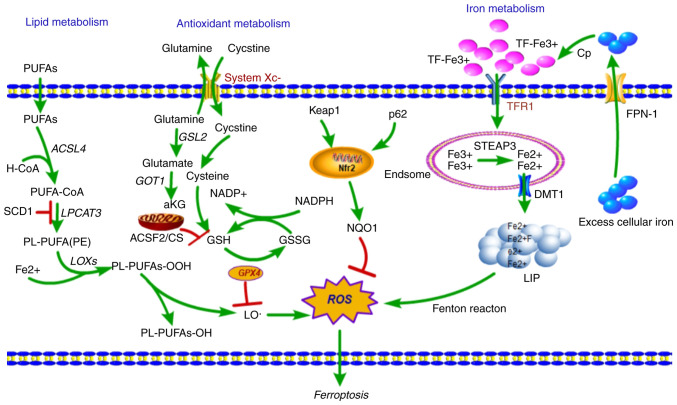
Figure 1.
Overview of the mechanism of ferroptotic cell death. Fe3+ is loaded into the circulating apo-Tf, forming a TfR1-Tf-(Fe3+)2 complex, which is endocytosed by TfR1, and iron is released from TF at same time. Fe3+ is reduced to Fe2+ by the ferric reductase STEAP3, and Fe2+ is then transported to the cytosol by DMT1, where it enters the cytosolic LIP for various metabolic needs. Excess iron is effluxed into circulation by FPN-1 and an associated ferroxidase, which causes the production of ROS, in-turn initiating ferroptosis. Lipid metabolism: Fatty acids are activated (ACSL4) and esterified (LPCAT3) into PL-PUFAs, then LOXs catalyze the dioxygenation of PL-PUFAs and generate PL-PUFAs-OOH. Lipid-OOHs are regulated by the balance of GPX4 activity. An excess of PUFAs enhances generation of ROS and toxic lipid peroxides and simultaneously decreases GPX4 activity, which initiates ferroptosis. Ferroptosis-related amino-acid metabolism: System Xc- imports cystine in exchange for glutamate, which is reduced to cysteine and used to synthesize GSH, a necessary cofactor of GPX4 for eliminating ROS. GSH is an antioxidant particularly important in protecting cells from ferroptosis. TfR1, Transferrin receptor 1; TF, Transferrin; LIP, labile iron pool; DMT1, divalent metal transporter 1; GPX4, glutathione peroxidase 4; STEAP3, six transmembrane epithelial antigen of the prostate 3; FPN-1, ferroportin 1; ROS, reactive oxygen species; PUFA, polyunsaturated fatty acids; LOXs, lipoxygenases; GSH, glutathione.