Figure 5. RACK1 Mediates NLRP3 Inflammasome Activation Independent of Its Ribosomal Binding Activity.
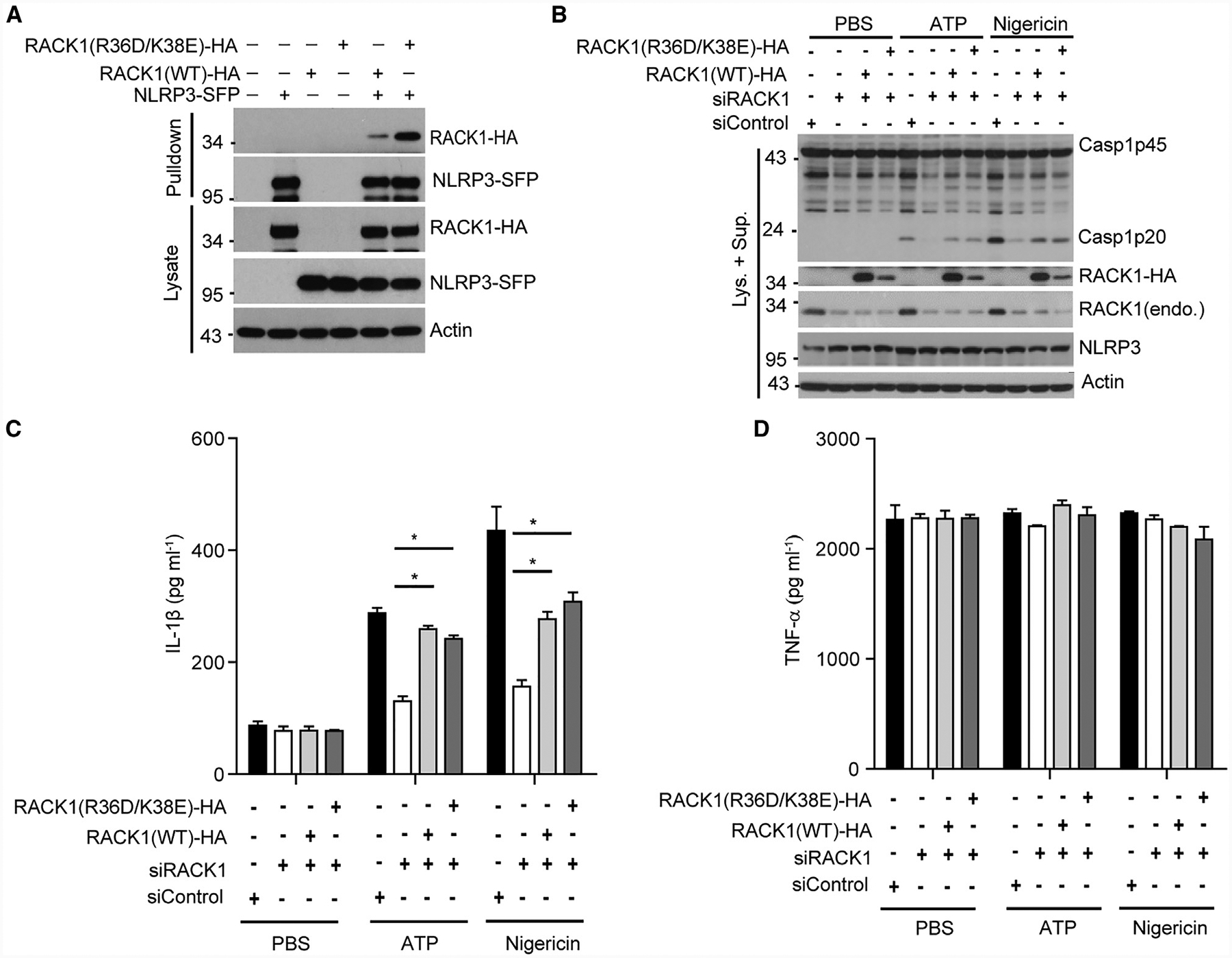
(A) Tagged NLRP3 (NLRP3-SFP) was co-expressed with HA-tagged wild-type RACK1 or mutant RACK1 (R36D/K38E, defective in ribosome binding) in HEK293T cells. Cell lysates were pulled down with streptavidin beads and analyzed by immunoblotting.
(B–D) Mouse macrophages were treated with RACK1 siRNA (siRACK1), followed by transduction with lentivirus expressing HA-tagged, siRNA-resistant, wild-type RACK1 or mutant RACK1 (R36D/K38E). Macrophages were primed with LPS (200 ng mL−1, 4 h) and then stimulated with PBS (control), ATP (5 mM, 30 min), or nigericin (5 μM, 1 h). Mixtures of cell lysates and culture supernatants were analyzed by immunoblotting with the indicated antibodies (B). IL-1β (C) and TNF-α (D) in culture supernatants were analyzed by ELISA. Error bars denote SD of triplicate wells. Results are representative of three independent experiments. Unpaired two-tailed Student’s t test, *p < 0.05.
